2013 年 30 巻 p. 267-275
2013 年 30 巻 p. 267-275
Recent improvements in precursor chemistry, reactor geometry and run conditions extend the manufacturing capability of traditional flame aerosol synthesis of oxide nanoparticles to metals, alloys and inorganic complex salts. As an example of a demanding composition, we demonstrate here the one-step flame synthesis of nanoparticles of a 4-element non-oxide phosphor for upconversion applications. The phosphors are characterized in terms of emission capability, phase purity and thermal phase evolution. The preparation of flame-made β-NaYF4 with dopants of Yb, Tm or Yb, Er furthermore illustrates the now available nanoparticle synthesis tool boxes based on modified flame-spray synthesis from our laboratories at ETH Zurich. Since scaling concepts for flame synthesis, including large-scale filtration and powder handling, have become available commercially, the development of industrial applications of complex nanoparticles of metals, alloys or most other thermally stable, inorganic compounds can now be considered a feasible alternative to traditional top-down manufacturing or liquid-intense wet chemistry.
Nanoparticle powder technology is a widely applied industrial process for the preparation of advanced functional materials (Hosokawa, 2008). Pre-designed and engineered nanostructures attract the interest of research and industry communities. Various methods of synthesis and control of these objects are constantly being developed and appear in high-impact journals, symposia and discussions. Despite a dramatic gap between academic methods for laboratory synthesis and the technological implementation on an industrial scale, various materials based on nanotechnologies are already available from large-scale production. Among the most promising methods are: CVD process for carbon nanotubes (Cassell et al., 1999); emulsion methods for polymeric nanoparticles (Muller et al., 2006) in drug delivery applications (PLGA, (Musyanovych et al., 2008)); sol-gel, e.g. LiFePO4 (Lee et al., 2010); precipitation, e.g. BaSO4, (Adityawarman et al., 2005); and hydrothermal methods (Chen et al., 2009) for various nanoparticles. However, the aforementioned methods are often inapplicable for metastable phases which often possess high functional characteristics.
Gas-phase synthesis stands aside as an independent process to fabricate nanopowders, particularly with aerosol methods. This technique allows a multi-scale and cheap production of nanoparticles. It is a relatively flexible technology by which also metastable phases can be obtained. Flame pyrolysis is currently applied in the large-scale production of SiO2 and TiO2 nanoparticles. The method is also promising for the production of nanostructured carbonates, e.g. CaCO3 (Huber et al., 2005) and SrCO3 (Strobel et al., 2006), sulfides, e.g. PbS and ZnS (Athanassiou et al., 2010), highly reactive metals, e.g. Co (Grass and Stark, 2006) and Ni (Jung et al., 2005), alloys, e.g. Cu-Ni (Jung et al., 2003), glasses, e.g. SiO2–CaO–P2O5–Na2O (Brunner et al., 2006), and halides, e.g. NaCl, BaF2, and CaF2,(Grass and Stark, 2005). An overview of nanoparticles prepared by flame-spray pyrolysis is shown in Fig. 1. The established materials, namely spherical oxide nanoparticles, a straightforward product of gas-phase synthesis, stand at the origin of the graph. This group typically includes titanium, silicon, and aluminum oxides. There has been evidence for more complex materials prepared by flame pyrolysis, e.g. Sr5(PO4)3Cl:Eu2+ (Kang et al., 2003). A reducing atmosphere helps to step away from oxidic nanoparticles towards metallic compounds. Acetylene-fed flames form carbon shells on the surface of metallic nanoparticles (Grass et al., 2007). Such carbon coatings allow chemical functionalization strategies of the nanoparticle surface to use them in filtration and purification (Rossier et al., 2011), magnetic chemical reagents (Wittmann et al., 2010) and catalysts (Zeltner et al., 2011). Sulfides derived from flame pyrolysis belong to another group of widely applied chalcogenide salts. Recently, Athanassiou et al. successfully prepared doped ZnS:Mn2+ nanoparticles (Athanassiou et al., 2010).
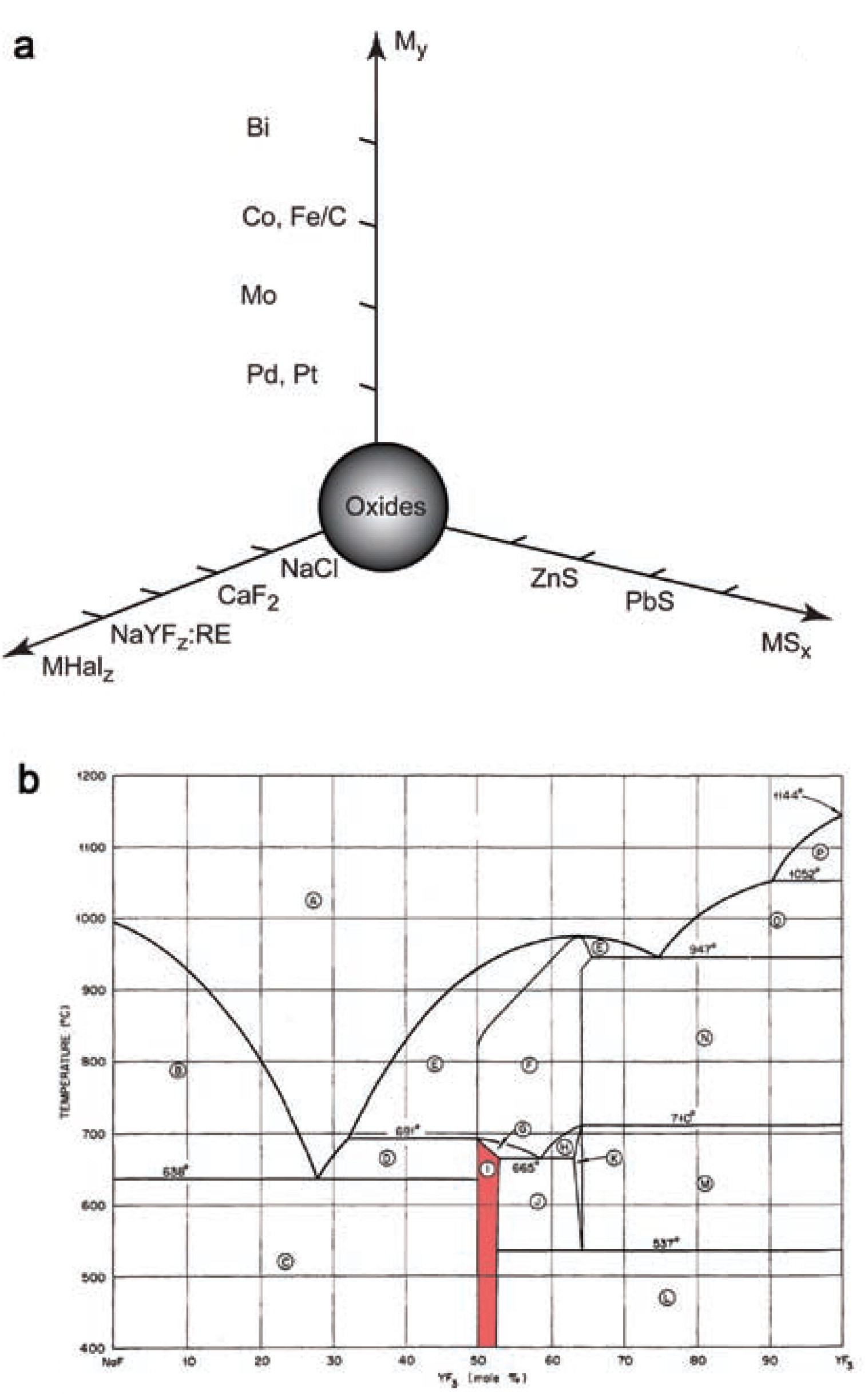
(a) Schematic representation of various nano-structures currently available by the flame-pyrolysis technique and (b) phase diagram of the NaF-YF3 system (Thoma et al., 1963). The shaded area marks the hexagonal NaYF4 stability region. Adapted with permission from Thoma et al., 1963. Copyright 1963 American Chemical Society.
Another group of industrially valuable non-oxidic salts are halides. Chlorides and fluorides of alkaline and alkaline-earth metals have been successfully obtained by gas-phase synthesis yielding nanoparticles of various morphologies and structures. Fluorides have unique optical and electronic properties which are widely used in biomaterials and electronic applications, though the need of more complex phosphors requires the preparation of glasses and mixed fluorides.
Recently, upconversion (UC) phosphors based on the rare earth and alkaline-earth materials have been investigated. In a UC process several low energy photons are absorbed, their energy is converted and finally a photon of higher energy is emitted. UC is also known as anti-Stokes emission (Auzel, 2004). It is known for f-elements such as the lanthanide ions Er3+ and Tm3+, as well as U3+, and several d-element ions embedded into specific matrices (Auzel, 2004).
Upconversion phosphors such as β-NaYF4: Yb, Tm or Yb, Er are regularly synthesized as bulk microcrystalline materials by high-temperature solid-state synthesis:
| (1) |
Lim et al. (Lim et al., 2009) successfully produced sub-10-nm polydisperse UC nanophosphors by flame-spray pyrolysis in a one-step continuous synthesis. The host matrix used for their study was cubic Y2O3 which is less efficient than hexagonal NaYF4 (Auzel, 2004). Grass et al. (Grass and Stark, 2005) proposed a fluoride doping with rare earth elements by flame synthesis. As a result, it appeared feasible to synthesize upconverting sodium yttrium fluoride nanoparticles doped with Yb-Tm or Yb-Er, although the cubic phase might be obtained instead of the hexagonal phase which shows better UC properties. Due to the limited thermodynamic stability of the hexagonal low-temperature phase, see the phase diagram in Fig. 1b, the cubic high-temperature phase of NaYF4 might be obtained from the synthesis as metastable kinetic product.
In the present work, flame pyrolysis is used to prepare nanoparticles of non-oxidic, doped rare earth fluorides. The upconversion emission during excitation with NIR laser and the thermal behavior of the derived phosphors are analyzed. The cubic-to-hexagonal phase transition of NaYF4 will be examined for various syntheses and thermal treatment conditions.
Powders of nano-sized upconversion particles (UCNP, NaYF4: Yb,Tm) were fabricated by flame-spray pyrolysis. Precursors of yttrium, ytterbium, erbium, and thulium were prepared from rare earth acetates Tm(CH3CO2)3·xH2O, Y(CH3CO2)3·xH2O (Fluka, 99.90% metal trace), and Yb(CH3CO2)3·xH2O (Acros Organics, 99.90% metal trace) by refluxing them with 2-ethylhexanoic acid (Fluka, puriss.) (Stark et al., 2003, 2004, 2005) and removal of acetic acid, for experimental conditions see Table 1. In the case of erbium-doped phosphors, Er(CH3CO2)3·xH2O (Fluka, 99.90% metal trace) was dissolved in 2-ethylhexanoic acid and the solution was directly combusted in the flame. NaHCO3 (Ph Eur, Applichem Co.) was mixed with 2-ethylhexanoic acid to obtain the sodium precursor. Each precursor solution was diluted with xylene (technical grade) to adjust the metal content to 1M and was filtered before combustion.
| Salt ion | Temperature, °C | Distillation time, hours | Concentration, wt. % |
| Y3+ | 130 | 6 | 6.3 |
| Yb3+ | 110 | 12 | 8.32 |
| Tm3+ | 120 | 2 | 0.7 |
The prepared stoichiometric mixtures of rare earth ethylhexanoates, sodium 2-ethylhexanoate, and fluorobenzene (ABCR-Chemicals, 99%) were combusted to produce NaYF4 nanoparticles, doped with 25 mol.% Yb and 0.3 mol.% Tm, i.e. NaY0.747Yb0.25Tm0.003F4, and 20 mol.% Yb and 2 mol.% Er, i.e. NaY0.78Yb0.2Er0.02F4. The solution was pumped through a 0.4-mm-diameter capillary at rates of 3, 5, 7, and 9 L·min−1 into fuel flames. Alternatively, the liquid solution was dispersed into an aerosol and burnt in oxygen (99.8%, Pan Gas) at rates of 7, 5, and 3 L·min−1 with a pressure drop at the capillary tip of 1.5 bar. A steady combustion was achieved by an oxygen (99.8%, Pan Gas) sheath gas flow of 230 l h−1 through a concentric sinter metal ring. A Teflon filter was used to collect the prepared particles of UCNP. The particles remained stable at ambient conditions. In the case of the reducing flame pyrolysis, the precursors were burned in a nitrogen-rich atmosphere using a nitrogen (5N, PanGas) glove-box with gas flow (Grass et al., 2007). This flow was circulated by a vacuum pump (Busch, Seco SV1040CV). The oxygen concentration was fixed below 100 ppm (volumetric) for the reducing flame pyrolysis.
Characterization of UC phosphorsThe specific surface area was calculated by measuring the nitrogen adsorption at 77 K on a Tristar (Micromeritics Instruments) following the Brunauer–Emmett–Teller (BET) method. Prior to the surface area determination, samples were preheated in vacuum at 150°C with p < 0.1 mbar during 1 hour.
The prepared NaYF4 nanoparticles with Yb-Tm and Yb-Er rare earth dopants were sintered at various temperatures (500 – 800°C) in air or nitrogen flow during 2 or 3 hours at heating rates of 10°C min−1. A “fast” heating of the NaYF4:Yb, Er upconversion phosphors was achieved by placing the powders directly in an 800°C preheated furnace.
The relative upconversion luminescence was measured according to the following procedure: Powders of the UC phosphors were filled in glass tubes of 1 mm inner and 1.5 mm outer diameter and then fixed in a sample holder. The powder densities were the same for all samples, as the powders were pressed into the glass tubes with glass tips. The samples were excited by a 980-nm IR laser diode coupled to a 1-mm-diameter fiber. The non-focused laser beam illuminated the sample in a spot of about 1 mm2 at a distance of 2–3 mm from the surface. The UC emission was collected by a Y-fiber, i.e. parallel to the excitation light, and measured by an Ocean Optics SD1000 spectrometer. The reflected IR laser light was blocked by a filter in front of the spectrometer. The laser power was measured by a power meter. The luminescence spectra were corrected for the spectral response of the detection system. The integrated emission peaks yielded the relative UC efficiencies of the powder samples with a reproducibility of 5%.
The size and shape of the as-prepared upconversion nanoparticles were characterized by transmission electron microscopy (TEM) with a Philips CM30 ST (LaB6 cathode, operated at 300 kV, point resolution 4 Å). Morphologies were also analyzed by scanning electron microscopy (SEM) with a Zeiss LEO 1530 Gemini. The phase composition was characterized by X-ray diffraction (XRD) patterns recorded with a PANalytical XPert PRO-MPD (CuKα radiation, X’Celerator linear detector system, step size of 0.033°, ambient conditions). The mean crystallite size was estimated from X-ray diffraction patterns by the Scherrer equation.
NaYF4: Yb, Tm powders were prepared by flame-spray synthesis. The obtained nanoparticles had diameters of 20–40 nm. The fuel/oxygen flow rates of 3/7, 7/3, and 7/9 L/min resulted in the formation of cubic α-NaYF4 particles, see Fig. 2a. Equation (2) shows the reaction scheme,
| (2) |
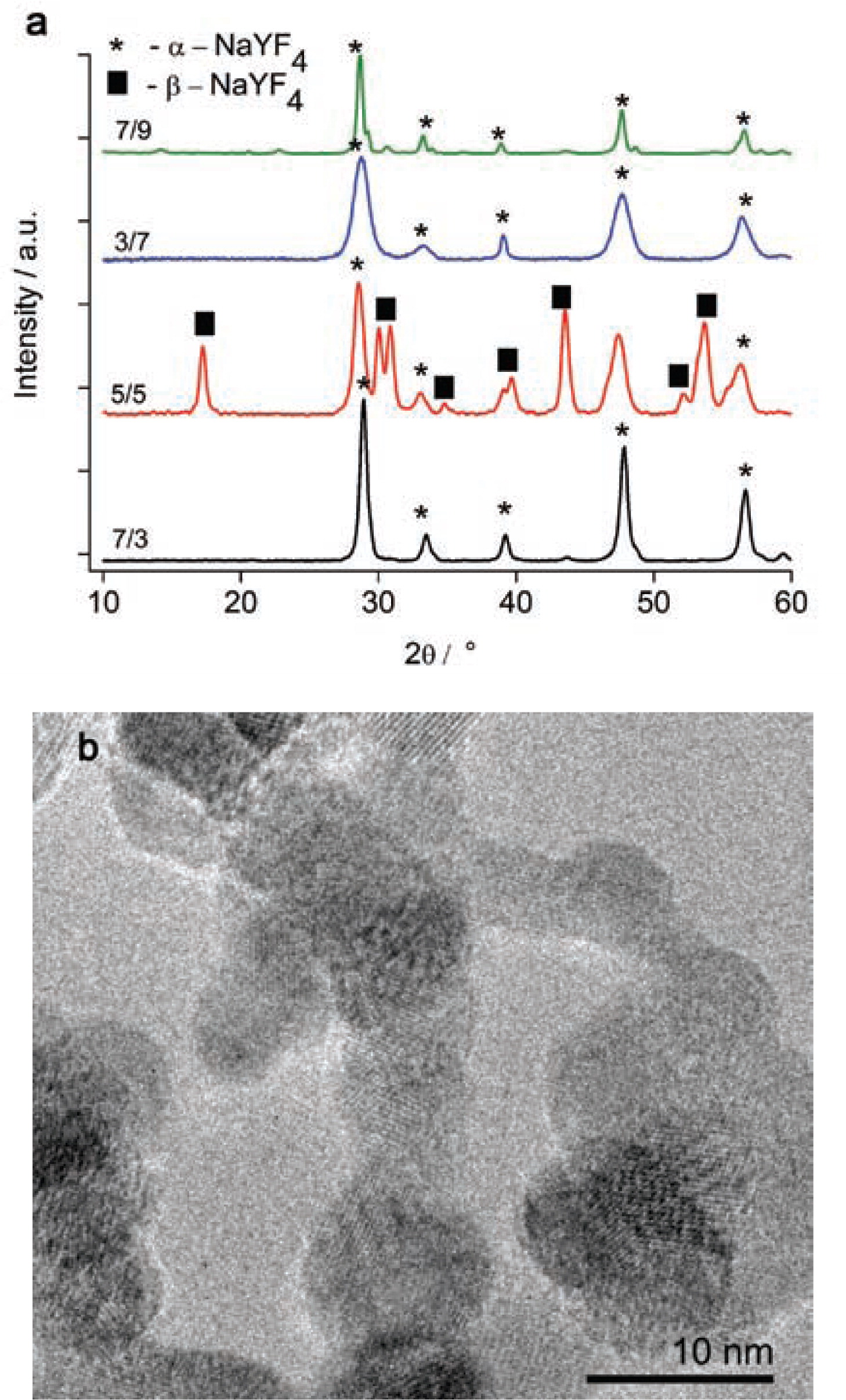
(a) X-ray diffraction patterns of NaYF4:Yb,Tm nanoparticles from different fuel/oxygen flow rates and (b) Transmission electron microscopy image of hexagonal NaYF4:Yb,Tm nanoparticles obtained from fuel/oxygen flow rates of 5/5 L/min.
The medium 5/5 L·min−1 feeding rate led to a mixed product with hexagonal(β) and cubic(α) phases of sodium yttrium fluoride, see Fig. 2a.
The TEM analysis of this powder revealed the presence of hexagonal particles. The particles have sizes of about 10 nm with agglomerates up to 50 nm, see Fig. 2b.
By the BET method, specific particle surface areas from 29.5 to 31 m2 g−1 were determined for nanoparticles prepared in oxidic or reduced atmospheres.
The calculated particle diameters according to equation (3) were 47 and 45 nm, respectively,
| (3) |
The SEM micrographs of flame-sprayed NaYF4: Yb, Tm also demonstrated the homogeneous distribution of nanoparticles without any visible micron-sized agglomerates, see Fig. 3a. These particles are stable at room temperature under atmospheric conditions. Images of the as-prepared NaYF4:Yb,Er nanoparticles show agglomerated clusters in the 100-nm range with hexagonal crystallites of less than 50 nm in size, see Fig. 3b. It correlates to previously measured BET and TEM particle size estimations. During sintering, the grains grow and micron-sized cubic crystals of Y2O3 form, see Fig. 3c. The image of a fragment in Fig. 3d shows narrow grain boundaries of β-NaYF4 crystals after 3 hours sintering at 700°C under constant nitrogen flow.
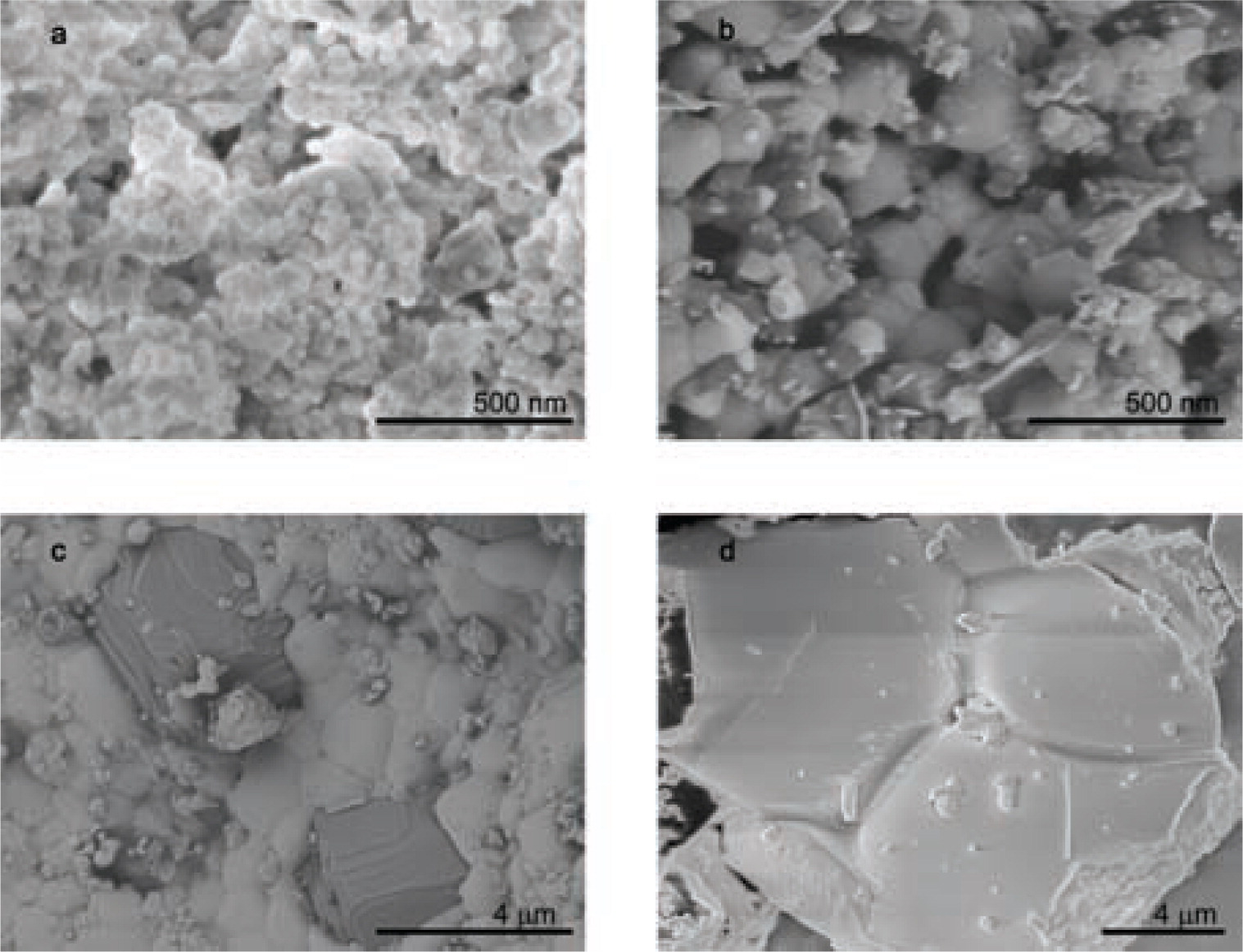
SEM micrographs of sodium yttrium fluorides prepared by the flame-spray method. (a) and (b) show highly anisotropic hexagonal nanoparticles of NaYF4: Yb, Tm and Yb, Er, respectively. (c) and (d) show images of micron-sized crystallites of NaYF4 and cubic Y2O3, respectively, after sintering at 700°C in ambient atmosphere.
The thermal behavior of the synthesized powders was studied by differential thermal analysis (DTA), see Fig. 4a. At low temperatures, the adsorbed water was released from the sample according to a weight loss of 0.3 wt% In addition, the DTA signal indicates a minor exothermal peak at 380°C It may correspond to the formation of traces of cubic yttrium oxide. At 400–500°C, the TG curve shows a step correlated to an endothermic peak on the DTA curve.
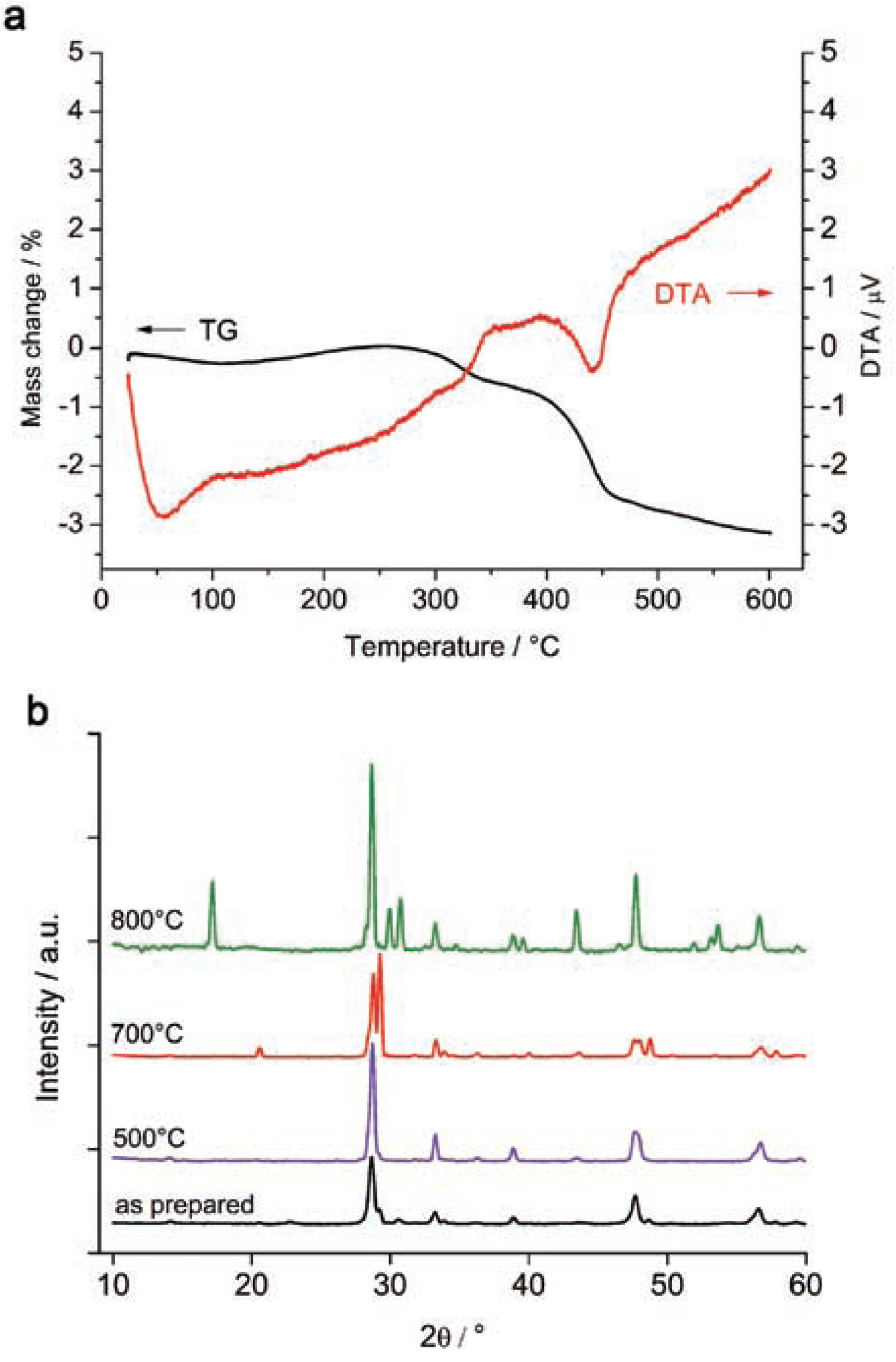
(a) Differential thermal analysis (DTA) and thermal gravimetric (TG) signals of NaYF4:Yb, Tm nanoparticles and (b) XRD diffraction patterns for different sintering temperatures of NaYF4:Yb, Er. 500°C and 700°C sintering was applied at a 10°C min−1 heating rate. For 800°C, a “fast” heating is achieved by placing the powder directly into the preheated furnace.
Analysis of the phase composition of upconversion phosphors sintered at 500°C showed decreasing half-widths of the Bragg peaks which correspond to an increased size of the crystallites. The NaYF4: Yb, Tm powders were sintered at temperatures from 500°C to 800°C. The XRD curves up to 700°C give no hint for a phase transition, see Fig. 4b. The sintering at 800°C shows a partial transition of sodium yttrium fluoride from its cubic to the hexagonal phase. According to the NaF-YF3 (Thoma et al., 1963) phase diagram, see Fig. 1b, the hexagonal β-NaYF4 phase is stable at room temperature whereas the high-temperature cubic α-phase is stable above 691°C. Thus cubic NaYF4 is obtained in the synthesis as a metastable, kinetically stabilized phase. Only at high enough temperatures, close to the α-β phase transition, can the α-phase overcome the activation energy barrier and transform into the β-phase. The ‘800°C’ curve in Fig. 4b shows a partial α-β phase transition. The sample temperature had obviously not been 800°C in this experiment, because then no β-phase would be obtained at all, but it had been high enough to partially activate the transition.
The thermal treatment at 700°C in an air flow during two hours led to the formation of Y2O3. This hydrolysis results in HF gas evolution and leaves Y2O3 and NaF behind, cf. equation (4).
| (4) |
In order to transform the cubic phase towards the more favorable hexagonal phase, the powders were sintered in vacuum. The X-ray diffraction patterns of samples treated at 500°C demonstrate the growth of crystals, see Fig. 4b, but no formation of hexagonal NaYF4. Crystallite sizes derived from the Scherrer Equation show a growth from 40 nm to 80 nm. Sobolev et al. (Sobolev et al., 1963) earlier demonstrated that the NaYF4 hexagonal phase undergoes a phase transition into NaYF4 with cubic symmetry at 600°C.
Further examinations of the flame-spray synthesis conditions and thermal treatment modes such as cooling rate are required. Flame pyrolysis allows the synthesis of nanocrystalline upconversion phosphors with a high surface area which show luminescence (Fig. 5b, 6a and b).

Upconversion luminescence emission of NaYF4 with Yb-Er (a) and Yb-Tm (b) rare earth dopant couples. The relative peak intensities increase after sintering from 500°C to 800°C.
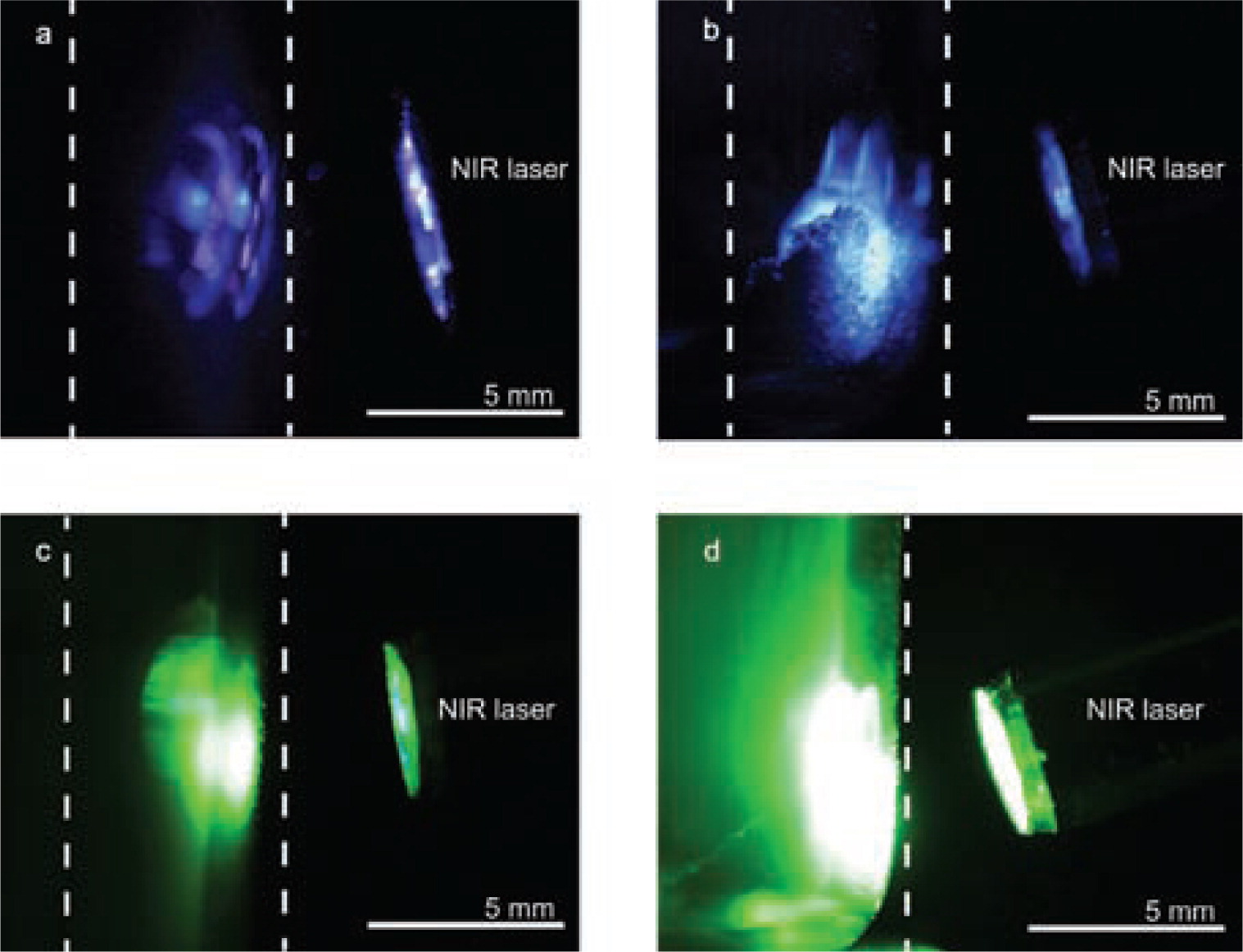
Photographs of the UC luminescence of Yb3+, Tm3+ (a, b) and Yb3+, Er 3+ (c, d)-doped nano-phosphors. The thermal treatment of powders at 800°C significantly increased the intensity of the visible emissions for the Yb3+, Tm3+ (b) and the Yb3+, Er3+ (d) phosphors.
NaYF4:Yb, Er samples were prepared using a 7/9 (L /min) feeding rate. The particles revealed green and red luminescence on excitation with a 980-nm IR laser. After sintering at 500°C and 700°C, the luminescence intensity increased because the crystallites grew in size. Fast heating, i.e. placing powders into a preheated furnace at 800°C, resulted in rapid formation of hexagonal sodium yttrium fluoride, see Fig. 4b. As a result, the green emission intensity increased, see Fig.5a. Phosphors sintered under an inert atmosphere formed oxide phases, which in turn intensified the red emission in the UC luminescence spectrum, see Fig. 5a. Yttrium oxide formation in the inert atmosphere is based on phase kinetics and prior built-in oxygen. This embedding happens during flame pyrolysis in an oxygen-containing environment. The formation of micron-size crystallites is observed on the SEM micrographs, see Fig. 3c. A slow heating rate of 10°C min−1 yielded a mixture of yttrium oxide and cubic NaYF4; the fast heating leads to hexagonal NaYF4 formation.
The luminescence spectra of upconversion phosphors doped with Yb3+ and Tm3+ show two characteristic visible emissions upon 980-nm excitation, see Fig. 5b. The red emission originates from the 1G4 → 3F4 transition. The blue emission consists of the two neighboring transitions of 1G4 → 3H6 and 1D2 → 3F4. Upconversion luminescence spectra also show emissions in the ultraviolet range ( 1D2 → 3H6 and 1I6 → 3F4 ). Higher sintering temperatures increase the luminescence intensity because larger crystallites have a smaller surface-to-volume ratio and therefore less defects (Suyver et al., 2006). The Yb3+, Er3+-doped α-NaYF4 shows a significantly higher red ( 4F9/2 → 4I15/2 ) than green ( 4S3/2 → 4I15/2 ) emission, see Fig. 5a. However, for the “fast heating” at 800°C sample, the green-to-red emission ratio becomes higher because hexagonal β-NaYF4 formed which has a significantly stronger green emission. Photographs of the UC emission of the respective samples are shown in Fig. 6 .
Pre-designed complex nanostructures with characteristic properties are built up by assembling their functional units step by step. In this regard, gas-phase methods have a great potential for industrially realizing some of the promises of nanotechnology. Their scaling potential and access to various types of precursors covers a plethora of nanostructures such as metals, oxides, salts, and complex compounds. In the present work, a successful bottom-up access through a refined flame-spray technique provides access to complex non-oxide particles such as rare-earth-doped sodium yttrium fluorides. The co-doping with Yb-Tm and Yb-Er ion couples leads to blue and green upconversion luminescence, respectively. Particles of less than 50 nm in size were obtained under reducing conditions as cubic NaYF4. It was possible to tune a cubic to hexagonal phase transition by thermal treatment of the nanomaterial. A strongly enhanced UC luminescence intensity was observed for the β-NaYF4. Upconversion luminescence spectra showed a correlation between crystallite size (i.e. low surface area) and luminescence intensity. Oxide impurities reduced the green and increased the red UC emissions in Yb3+, Er3+-doped phosphors.
Alex Stepuk
Alex Stepuk received a BSc. in materials science at Moscow State University. He graduated with an MSc of the Materials Science Department at ETH Zurich with a pioneering thesis on the implementation of upconversion phosphors in dental photopolymers. He is currently a PhD candidatein the group of Prof. Wendelin J. Stark at the Department of Chemistry and Applied Biosciences at ETH Zurich. His research interests are interdisciplinary, covering applications of flame-spray-derived nanoparticles in dental materials and orthopedics, materials for energy conversion and polymers in medicine.
Karl W. Krämer
Karl W. Krämer received his chemistry diploma (1988) and his Dr. rer. nat. (1991) from the Justus-Liebig University, Giessen, Germany. He is the group leader of solid state analytics at the Department of Chemistry of the University of Bern, Switzerland. His research focuses on the synthesis of anhydrous metal halides, their crystal growth, and investigation of spectroscopic and magnetic properties. Recent topics are upconversion phosphors, e.g. β-NaYF4:Yb,Er or Yb,Tm, Ce3+-doped scintillators, e.g. LaBr3:Ce, and quantum spin ladders, e.g. [piperidinium]2CuBr4.
Wendelin J. Stark
Wendelin J. Stark received his master’sdegree in chemistry in 2000, followed by a PhD in mechanical engineering in 2002, both from ETH Zurich. In 2004, he founded the Functional Materials Laboratory within the Departments of Chemistry and Applied Bioscience at the ETH Zurich. His research group pursues application-oriented research at the interface of chemistry with materials science and medicine.