2019 年 36 巻 p. 224-231
2019 年 36 巻 p. 224-231
Uniform spherical graphene/monocrystal-copper powder is fabricated by melting bulk laminated composite. Graphene, in the form of reduced graphene oxide (rGO), is uniformly dispersed on the surface of monocrystal Cu particle. A mechanism is proposed based on liquid/solid interface interaction combining the surface energy minimization of liquid Cu and low wettability of molten Cu on graphene. The rGO/Cu composite powder exhibits good sphericity, and the size distribution could be controlled by the amount of rGO.
Particle morphology control is fundamental in the processing of metal powders, such as 3-D printing, spraying, and injection moulding (Attia U.M. and Alcock J.R., 2011; Das S. et al., 2016; Lampke T. et al., 2011). Powders with good sphericity and controllable particle size are usually required. The most commonly methods used in fabricating metal powders are based on general surface tension principles at the liquid/gas or liquid/liquid interfaces, such as gas atomization (Ünal A., 1987), pressure-gas-atomization (Achelis L. and Uhlenwinkel V., 2008), water atomization (Seki Y. et al., 1990), and spark erosion in a dielectric fluid (Berkowitz A.E. et al., 2004). Recently, Lei et al. proposed a new method based on liquid/solid interface to prepare Cu-Zn powder (Lei C. et al., 2015). However, most of the methods mentioned above are developed for metal or alloy powders, and that for composite powder is still largely unexplored. Graphene has attracted much attention owing to its excellent mechanical, electrical, and thermal properties.
Graphene has shown a promising potential in improving the mechanical properties of metals (Cao M. et al., 2017; Kim Y. et al., 2013; Xiong D.B. et al., 2015), and also graphene wrapped micro-/nano-structures exhibit enhanced electrical and thermal conductivity (Goli P. et al., 2014; Mehta R. et al., 2015). Additionally, graphene encapsulated nanoparticles show a competitive advantage in lithium-ion batteries (Zhang J. et al., 2013). Therefore, graphene/metal composite powder may have great potential in the applications of metal materials processed by the technologies of 3-D printing, spraying, and injection molding. However, fabricating the composite powders is still a challenge. In this article, we proposed a new method based on liquid/solid interface interaction to fabricate graphene/copper composite powder with good sphericity and controlled size distribution.
The rGO/Cu bulk laminated composites were fabricated based on a modified flake powder metallurgy (Cao M. et al., 2017). Atomized pure copper powders (99.99 % purity, with an average particle size of 44 μm) were ball-milled to Cu flakes in pure ethanol in a stainless steel mixing jar. The mass ratio of the powder to milling balls (100 % stainless steel milling balls with diameter of 6 mm) is 1:20. The ball milling speed is 423 rpm and the ball milling time is 5 h. The as-prepared Cu flakes (30 g) were coated with polyvinyl alcohol (PVA) for surface modification by stirring them in 250 mL 1 wt% PVA aqueous solution and subsequent centrifugation and washing with deionized water. Then the PVA modified Cu flakes were dispersed in 250 mL deionized water, and 18.0 mL (for 0.5 vol%) or 54.5 mL (for 1.5 vol%) GO aqueous suspension was added into the slurry. The GO suspension (1 mg/mL) with the lateral size of 400–500 nm and the thickness of 1 nm was prepared straight from ultrasound treating graphite oxide (Li Z. et al., 2014). The amount of GO added was calculated by characterizing the GO density to 1.06 g/cm3 (Rafiee M.A. et al., 2009).
After stirred for 1 h, filtered and then dried in vacuum at 60 °C, the as-obtained GO/Cu composite flakes were reduced under 5 % H2/Ar flow at 450 °C for 2 h. Finally, the bulk laminated composites were obtained by reducing the GO/Cu composite flakes (rGO/Cu) and assembling them in a graphite die (Φ30 mm), and then hot-pressed at 900 °C and 50 MPa for 20 min under Ar atmosphere.
2.2 Preparation of spherical rGO/Cu powderThe rGO/Cu bulk laminated composites were treated in a tube furnace in N2 atmosphere at 1100 °C for 30 min with a heating rate of 10 °C/min. The rGO/Cu powder was obtained by natural cooling to room temperature under N2 atmosphere.
2.3 Characterization of spherical rGO/Cu powderThe rGO/Cu powders were mounted in thermosetting resin and then polished by a vibratory polisher for microstructure and elemental analysis on cross section. Microstructure characterization was performed via field-emission scanning electron microscopy (FE-SEM, Sirion 200) equipped with an energy dispersive spectrometer (EDS, INCA X-Act) for compositional analysis. The electron backscatter diffraction (EBSD) was carried out in SEM (NOVA NanoSEM 230). The Raman spectra was performed at dispersive Raman microscope (Senterra R200-L) with a 532 nm laser excitation.
Fig. 1 shows the SEM image of the GO/Cu composite powder flakes, where GO sheets are uniformly dispersed on the surface of Cu flakes. The white folds (black arrows) are signs of the wrinkling and existence of graphene. Usually, the GO sheets are very thin and almost transparent, but the transferred or absorbed graphene on substrates has line defects and disruptions such as wrinkles, ripples and foldings. And the surface roughness is also different with or without covered GO. The area with covered GO (black circle) is smoother than without covered GO. Combining the two aspects, we can identify the GO sheets and their uniform dispersion on the Cu surface.
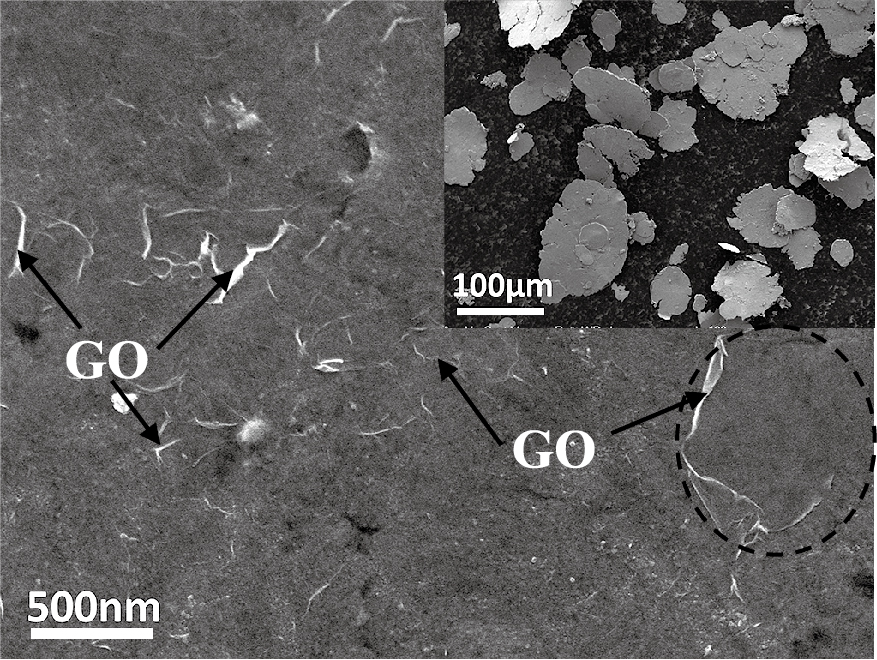
SEM micrographs of GO/Cu composite flakes, showing GO was uniformly dispersed on the surface of Cu flakes.
The GO/Cu composite flakes have large aspect ratio (Lateral size and thickness ratio is about 200–400), which is beneficial for forming laminated structure, as shown in the fracture surface of the rGO/Cu bulk composite (Fig. 2a). The existence of rGO prevents the Cu flakes from growing up perpendicular to the lamella during the hot-pressing process. As revealed by the fracture surface of pure copper fabricated by flake powder metallurgy in Fig. 2b, the Cu flake cannot hold its laminated structure due to grain growth during heat treatment. The homogenous laminated structure is crucial for fabricating uniform spherical rGO/Cu composite powder. And the degree of the homogeneity of the laminated structure mainly characterized by the homogeneity of lamellar distance. The more uniform the lamellar distance is, the more conducive to the preparation of the uniform rGO/Cu powder. The lamellar distance in Fig. 2a is relatively uniform, which is about 0.5 μm.

(a) SEM micrograph of the fracture surface of bulk rGO/Cu composites fabricated by assembling the flakes, showing a laminated structure. (b) SEM micrograph of the fracture surface of pure copper fabricated by flake powder metallurgy.
Fig. 3 shows the SEM images of rGO/Cu powders fabricated via melting the bulk laminated composites with rGO content of 0.5 vol% (Fig. 3a) and 1.5 vol% (Fig. 3b). The rGO/Cu powders exhibit good sphericity, and the degree of sphericity computed by image processing is 0.9 ± 0.1 for both two rGO content. The rGO sheets are homogeneously dispersed on the particle surface and the coverage rate is about 50 %–60 % for 0.5 vol% and >100 % (Graphene sheets overlap with each other in some extent) for 1.5 vol%. The distributions of particle size scaled by the diameter of the powders are shown in Fig. 3c and d. The average particle sizes (

(a)(b) SEM micrographs of the spherical rGO/Cu composite powders with GO fraction of (a) 0.5 vol% and (b) 1.5 vol%; (c)(d) particle size distribution of the spherical rGO/Cu composite powders with GO fraction of (c) 0.5 vol% and (d) 1.5 vol%.
Raman spectroscopy was employed to characterize the original GO and the rGO on the surface of the composite powder (Fig. 4). It is predicted that the D/G intensity ratio (ID/IG) of GO decreased after reduction. However, ID/IG of rGO (0.95 in Fig. 4b) is found to be larger than that of original GO (0.75 in Fig. 4a). One possible reason is that new created graphitic domains in rGO are more numerous in number, but smaller in size comparing with integrated GO. So, the average size of the sp2 domains decrease and the edge of sp2 domains greatly increase after reduction, which leads to the increase appearing in ID/IG (Paredes J.I. et al., 2009; Ren P.G. et al., 2011). Besides, it is reported that ID/IG firstly increases with increasing mean distance between defects (LD) from 1 nm to about 3 nm and then decreases when LD is larger than 3 nm (Cançado L.G. et al., 2011; Eigler S. et al., 2012). Thus, another possible explanation of ID/IG increase is that the LD of our sample increases after reduction but still within 3 nm.
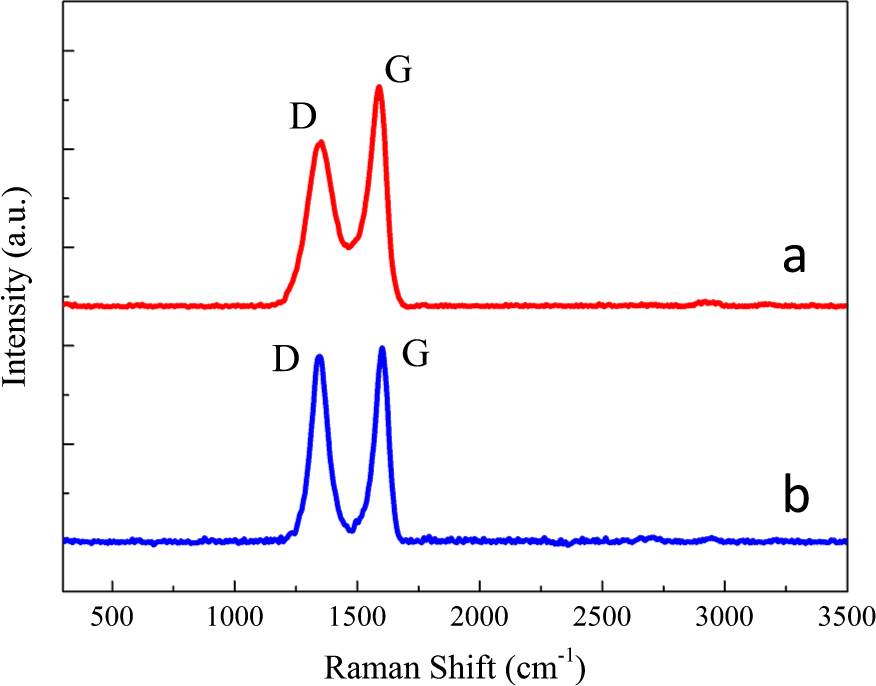
Raman spectra of (a) original GO and (b) rGO on the surface of rGO/Cu composite powder.
Microstructure and elemental analyses were performed on the surface area and cross-section of the rGO/Cu powder. The EDS analysis in Fig. 5(a) shows that both carbon element and copper element are detected on the surface of the powder but carbon element is majority, showing most of the surface area is covered with carbon element. The EDS result in Fig. 5(b) shows that only copper is detected inside the rGO/Cu particle, indicating that graphene only disperses on the particle surface, which is consistent with the SEM results (Fig. 3a, b). As revealed by the EBSD result (Fig. 6), the Cu particle in the composite particle is monocrystal with the same orientation. Therefore, the composite powder is depicted as spherical monocrystal Cu core encapsulated with reduced graphene oxide.
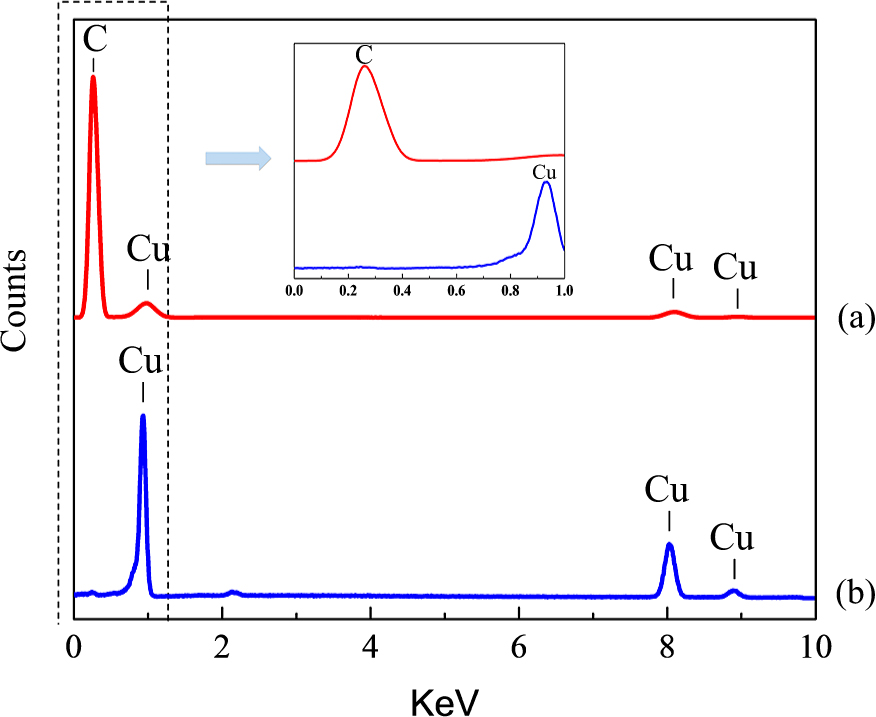
EDS spectra of the element analysis of the (a) surface and (b) the cross-section of the rGO/Cu composite powder, showing the rGO dispersed on the surface of the powder and there only exists Cu element inside the powder.
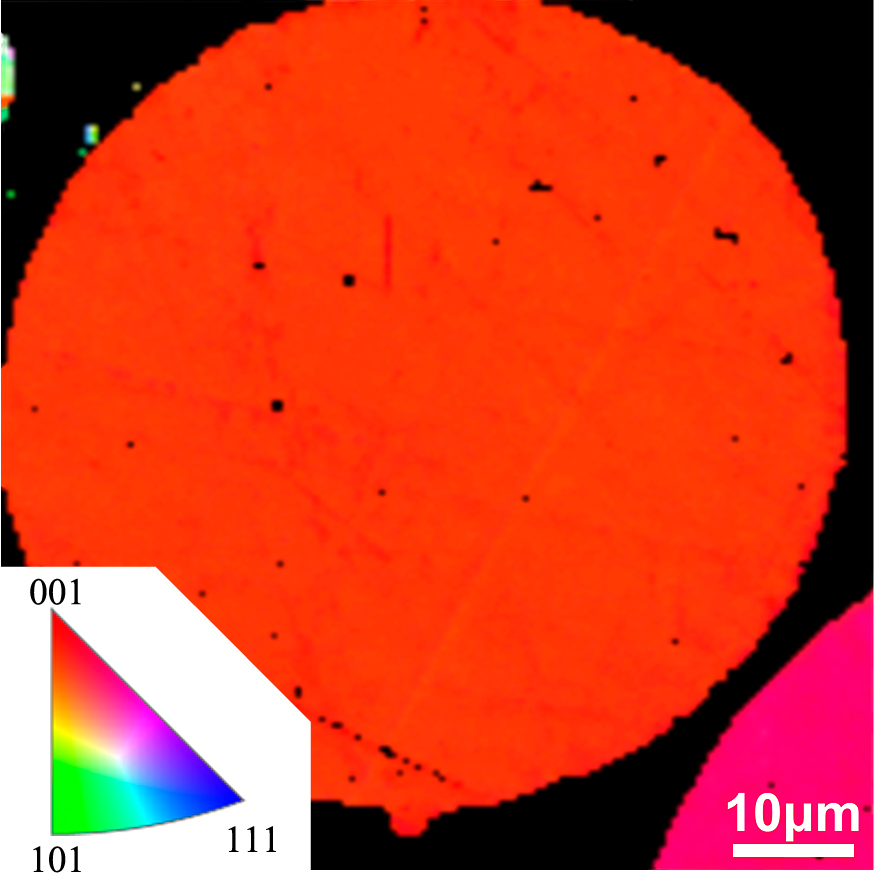
An EBSD image of the cross-section of the rGO/Cu composite powder, showing the Cu phase is monocrystal.
The spherical rGO/Cu composite powder is formed after the bulk laminated rGO/Cu composites (Fig. 7a) go through over-melting point treatment and cooling process, and a possible formation mechanism is illustrated schematically in Fig. 7. Copper (with a melting point of 1083 °C) would melt at the temperature up to 1100 °C, while the rGO sheet remains unchanged because carbon has an ultrahigh melting point and carbon and copper are immiscible as well as no chemical reaction between them.

Schematic illustration of the formation of spherical rGO/Cu composite powder.
Further, spherical particles form because of a poor wettability of pure liquid Cu on carbon and surface energy minimization of a droplet. The wetting or de-wetting depends on the physical properties of the liquid and solid phases, especially the surface and interfacial energy. The wettability can be expressed using the equilibrium contact angle (θ) (Fig. 7d) of a metal droplet on the solid surface, and it can be described by the Young’s equation:
| (1) |
where γs is the surface energy of solid phase, γl is the surface energy of liquid phase, and γsl is the interfacial energy between the solid phase and liquid phase. The surface energy γs of rGO is estimated to be between the surface energy of graphene (46.7 mJ/m2) and GO (62.1 mJ/m2), and closes to that of graphite (54.8 mJ/m2) (Wang S. et al., 2009), but much lower than that of pure liquid Cu (1400 mJ/m2 at the melting point) (Keene B.J., 1993). We assume that the equilibrium contact angle of rGO/Cu is similar to that of graphite/Cu (140° at 1150 °C) (Mortimer D.A. and Nicholas M., 1970), so the liquid Cu on rGO almost completely de-wets. Besides, surface energy minimization of a liquid Cu droplet drives the formation of spherical shape. After cooling down, the rGO sheets coat on the surface of the monocrystal Cu core. The contact between rGO and Cu should be dominated by a metallurgical bonding because graphene would not react with Cu even at high temperature under pressure (Cao M. et al., 2017).
In this work, we have fabricated spherical rGO/Cu composite powder based on the liquid/solid interface interaction, which takes advantage of low wettability of liquid Cu on solid rGO. The Cu phase of the composite powder is monocrystal and the rGO coats on its surface. The resulting composite powder exhibits good sphericity and narrow particle size distribution. Besides, it sheds light on fabricating other metal composite powders.
The authors would like to acknowledge the financial supports from the Ministry of Science & Technology of China (the National Key Research and Development Program of China 2017YFB0406200), and Shanghai Science & Technology Committee (Nos.14JC1403300, 14DZ2261204, 15JC1402100, 17520712400).
Average particle sizes
EBSDElectron backscatter diffraction
EDSEnergy dispersive spectrometer
FE-SEMField-emission scanning electron microscopy
GOGraphene oxide
IDIntensity of D-band
IGIntensity of G-band
LDMean distance between defects (nm)
PVAPolyvinyl alcohol
rGOReduced graphene oxide
σStandard deviation of diameter (μm)
θEquilibrium contact angle
γsSurface energy of solid phase (mJ/m2)
γlSurface energy of liquid phase (mJ/m2)
γslInterfacial energy between the solid phase and liquid phase (mJ/m2)
Jimin Lyu
Jimin Lyu received her Bachelor degree in Materials Science and Engineering from Shanghai Jiao Tong University in 2015 and currently is a master student at State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University. Her current research focuses on the fabrication and characterization of graphene reinforced copper matrix composites.
Ding-Bang Xiong
Ding-Bang Xiong is an Associate Professor in the State Key Lab of Metal Matrix Composites, in Shanghai Jiao Tong University, China since 2012. He received Ph.D. degree in material physics and chemistry from Shanghai Institute of Ceramics in 2007. Between 2007–2010, he carried out his postdoctoral research as Alexander von Humboldt fellow in Marburg University, Germany, and between 2010–2012, he did collaboration research in Kyoto University supported by the Japan Society for the Promotion of Science. His current research focuses on fabrication and properties of metal matrix composite materials.
Zhanqiu Tan
Zhanqiu Tan is an Assistant Researcher at State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University since 2016. He received his Ph.D. degree in Materials Science and Engineering from Shanghai Jiao Tong University (2014), where he studied particulates reinforced aluminum matrix composites with high thermal conductivity for thermal management applications. He completed his postdoctoral research on interface control and performance of Carbon/Aluminum composites in 2014–2016 at Shanghai Jiao Tong University. His current research focuses on high performance nano-Carbon (carbon nanotubes, graphene et al.) reinforced metal matrix composites.
Genlian Fan
Genlian Fan is an Assistant Professor at State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University since 2011. She received her Bachelor degree and Ph.D. degree in Materials Science and Engineering from Xi’an Jiaotong University. Between 2009–2011, she completed her postdoctoral research in Shanghai Jiao Tong University. Her current research focuses on smart powder metallurgy processing for fabrication of advanced metal matrix composites, carbon nanotube & graphene reinforced metal matrix composites, and defect relaxation & energy dissipation of metal matrix composites.
Qiang Guo
Qiang Guo is an Associate Professor in the School of Materials Science & Engineering and the State Key Lab of Metal Matrix Composites, in Shanghai Jiao Tong University, China since 2012. He received the B.Sc. degree in microelectronics from Peking University, China in 2005, and received the M.Eng. degree in materials science and engineering from Massachusetts Institute of Technology (MIT), in 2006. He obtained the PhD degree in advanced materials in 2010 from National University of Singapore, under the Singapore-MIT Alliance (SMA) program. Between 2010–2012, he was a postdoc in the Department of Applied Physics and Materials Science in California Institute of Technology (Caltech). His current research focuses on the mechanical behavior of advanced metal matrix composites.
Cuiping Guo
Cuiping Guo is an Assistant Engineer at State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University since 2009. She received her Bachelor degree in Mineral Processing Engineering from Central South University, China in 2006 and received Master Degree on research on the preparation and property of bio-sorbent made from agricultural residues in 2009 at Shanghai Jiao Tong University. Her Current research focuses on materials characterization.
Zhiqiang Li
Zhiqiang Li is a Professor at State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University. He received his Bachelor degree in 1996, Master degree in 1998 and Ph.D. degree in 2001 from Harbin Institute of Technology. Between 2002–2003, he completed his postdoctoral research in Shanghai Jiao Tong University and later worked in Shanghai Jiao Tong University. During 2011–2012, he did visiting research in Osaka University. His current research focuses on fabrication and mechanism of bionic composite materials, deformation of metal matrix composites, and powder metallurgy composite technology & principles.
Di Zhang
Di Zhang is a Professor in Shanghai Jiao Tong University and is the director of State Key Laboratory of Metal Matrix Composites. He is awarded by Ministry of Education in China as “Cheung Kong Scholars Program” Distinguished Professor in material subject. He started to teach at Shanghai Jiao Tong University in 1988 and became a Professor in 1994. He was a visiting scholar or visiting professor in Japan Yuasa Central Research Institute, Osaka University, Kyoto University, Germany Max-Plank Institute of Metals and Saga University, successively. His research focuses on metal matrix composites and genetic materials template from nature species.