2019 年 36 巻 p. 114-128
2019 年 36 巻 p. 114-128
Ceramics with highly controlled microstructures at all levels from micrometer to nanometer order are required to improve their properties. To realize such ceramics, advances in powder processing are indispensable. Powder processing involves the preparation of fine particles, surface modification, consolidation, and sintering. The colloidal processing using fine particles has received particular attention as a means of achieving ceramics with highly controlled microstructures. Here, the preparation of fine-grained ceramics through a well-dispersed suspension and the preparation of porous ceramics and nanocomposites through heterocoagulated suspensions are demonstrated. Then novel colloidal processing under an external field such as a strong magnetic field and/or an electric field and the fabrication of textured ceramics and laminated composites are demonstrated.
Precise control of the hierarchical, graded, laminated, and/or oriented microstructures at all levels from micrometer to nanometer order is required to add new functions and improve the performance of advanced ceramics. To satisfy the requirements for ceramics, advances in powder processing are indispensable (Sakka Y., 2006). Fine powder processing consists of the following processes: (1) preparation of fine particles, (2) surface modification of the fine particles, (3) consolidation, and (4) sintering. To obtain a fine microstructure after sintering, fine particles should be used as the starting material. However, as the particle size decreases, particles tend to agglomerate easily, leading to a nonuniform structure containing large pores caused by the agglomerates. To address this problem, colloidal processing, in which fine particles are dispersed in a solvent, formed, and consolidated, has been attracting attention (Lange F.F., 1989; Akasay I.A., 1991; Sakka Y., 2007).
If fine particles are densely packed in ceramic compacts and the distribution of the pore size is narrowed by controlling the dispersion of the fine particles, densification will occur at low temperatures, resulting in a dense and fine-grained microstructure. In addition, nanocomposites and ordered porous materials can be fabricated by the heterocoagulation of suspensions with well-dispersed fine particles having opposite charges. Furthermore, an electric field and a strong magnetic field applied externally during colloidal processing enable the high-level control of microstructures such as graded, laminated, and/or oriented microstructures.
As effective sintering methods for realizing a rapid temperature increase, microwave or millimeter-wave sintering and pulsed electric current sintering, in which pressure sintering is carried out while a pulsed current or voltage is applied to specimens (generally called spark plasma sintering (SPS), have been attracting attention (Grasso S. et al., 2009). In particular, SPS enables us to obtain compact and fine structures at a low temperature in a short time by applying pressure. Owing to the page limitation, novel sintering methods including SPS are not discussed in this review, but the understanding of the basic sintering mechanisms is very important (Maizza G. et al., 2007, 2009). Needless to say, for the development of high-performance ceramics, feedback from advanced analytical technology and simulations is crucial (Sakka Y., 2006).
This review discusses some merits of colloidal processing and demonstrates the fabrication of fine-grained ceramics using a well-dispersed suspension, and porous ceramics and nanocomposites through heterocoagulated suspensions. Then the fabrication of textured ceramics and laminated composites by novel colloidal processing under external fields such as a strong magnetic field and/or electric field is described.
In colloidal processing, fine particles are dispersed in a solution and consolidated to obtain a dense compact. Table 1 shows typical colloidal processing accompanied by a drain step, in which the transport media and their driving forces are indicated (Nicholson P.S. et al., 1997; Sakka Y., 2007).
| Method | Force | Actors | |
|---|---|---|---|
| Watchers | Movers | ||
| slip casting | capillarity | particles | liquid |
| both ions | |||
| pressure/vacuum casting | capillarity | particles | liquid |
| and/or pressure | both ions | ||
| and/or suction | |||
| centrifugal force casting | centrifugal force | both ions | particles |
| liquid | |||
| tape casting | mechanical | liquid | - |
| particles | |||
| both ions | |||
| EPD | electrohydrodynamics | liquid | particles |
| electrochemical | both ions | ||
Slip casting (Fig. 1(a)) is the most widely used method of colloidal processing; a suspension is poured into in a porous mold and a consolidated layer is obtained. The consolidation rate is controlled by the drain rate at which the solvent is removed through the consolidated layer formed by the packed particles. Therefore, the consolidation rate is slower when using fine particles. Pressure casting, vacuum casting, or centrifugal casting is useful for the consolidation of fine particles. In these methods, the solvent is removed and a porous mold is necessary.
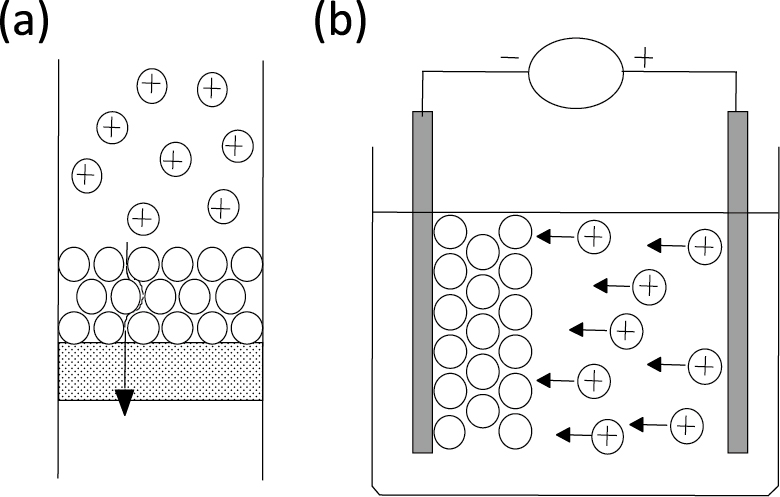
Schematic of slip casting (a) and EPD (b).
Tape casting is a method for forming a sheet film in which the suspension is poured onto a moving sheet via a knife edge, a so-called doctor blade. This process is widely used to form a laminated layer. In electrophoretic deposition (EPD), charged particles are moved and deposited on a substrate in an externally applied electric field. This method is suitable for the fabrication of ceramics with a highly controlled structure from fine particles because the deposition rate is high, regardless of the particle size, and the position and arrangement of the particles as well as the layer thickness can be controlled via the electric field (Fig. 1(b)) (Sarkar P. and Nicholson P.S., 1996; Besra L. and Liu M., 2007; Sakka Y. and Uchikoshi T., 2010).
As another colloidal processing without accompanying dehydration, gel casting and floc-casting have received increased attention because of isotropic shrinkage and precise dimensional accuracy (Young A.C. et al., 1991; Omatete O.O. et al., 1997; Santacruz I. et al., 2005; Bednarek P. et al., 2010). These methods are based on in-situ solidification by the polymerization of monomers or flocculation upon heating, etc., using a high-solid loading suspension. When using fine particles, such as a high-solid loading suspension cannot be prepared and a special technique is necessary for applying such a process.
The key to colloidal processing is to control the dispersion and the stabilization of fine particles in a solvent. Therefore, understanding of the characteristics of each particle in the solvent is essential. A ceramic particle is charged in a solvent, particularly in an aqueous suspension, owing to the particle surface (or surface adsorbed species) and solvent. During practical processing, systems where the particle dispersion can be controlled by the pH are limited; therefore, the adsorption of a polyelectrolyte with -COOH or -NH3 on the powder surface is usually conducted. In this case, electrosteric stabilization is expected owing to the surface charge of the electrolyte and the adsorption of the polymer (Cesarano J. et al., 1998; Zhu X.W. et al., 2003; Tang F.Q. et al., 2006). In general, fine particles tend to agglomerate and a redispersion treatment in a solvent is necessary.
For the redispersion of nanoparticles and heavily aggregated particles, bead milling using small beads with a size of 15–50 μm is effective (Inkyo M. et al., 2006; Suárez G. et al., 2009a,b; Ogi T. et al., 2017). Fig. 2 shows fine particles of hydroxyapatite (HAP) with a mean size of 48 nm, heavily aggregated HAP particles with a mean size of 280 nm after ultrasonic irradiation, and HAP particles with a mean size of 280 nm after milling using zirconia beads with a size of 50 μm. From the figure, it can be seen that the dispersion of the fine particles and heavily agglomerated particles by ultrasonic irradiation is difficult; however, it is possible by bead milling. A dense compact with a narrow distribution of pore size was fabricated by the pressure filtration of a suspension of fine HAP particles. After sintering at 1000 °C, dense and fine grained HAP with a mean grain size of 170 nm was successfully fabricated (Fig. 3).

HAP fine particles with a mean size of 48 nm (upper), heavily aggregated HAP particles with a mean size of 280 nm (lower) after ultrasonic irradiation (left), and those after bead milling (right).

SEM photo of fine grained HAP after sintering at 1000 °C, and photos before and after superplastic deformation.
In ceramic materials in which the grains are rigid, the combination of grain-boundary sliding, grain switching and grain rearrangement due to diffusion (Ashby M.F. and Verrall R.A., 1973; Gifkins R.C., 1978) can be regarded as the main mechanism of superplastic deformation. If this combination is ideally uniform and successive, then superplastic deformation is also uniform and successive without cavitation damage along grain boundaries or at multiple junctions. For such an ideal case, the stress– strain rate relationship is given by
| (1) |
Here,
Therefore, the factors contributing to the superplastic deformation in monolithic ceramics are the refinement of the grain size, the decrease in the initial defect density, and a homogeneous microstructure (Sakka Y. et al., 2001; Hiraga K. et al., 2007). These are the merits of colloidal processing using fine particles.
When the strain rate was 5.56 × 10−5/s at 1100 °C, the tension extension of the fine-grained and dense HAP reached the maximum value of 220%, and the superplasticity (above 200% elongation) was achieved in HAP for the first time. Fig. 3 also shows the shape of the test specimen before and after the tensile test.
Fig. 4 schematically shows the relationship between the pH and zeta potential of components A and B, and the dispersion state of both components. In regions b and d in the figure, the flocculation of one component occurs owing to a low zeta potential, and the dispersion state cannot be established, as schematically shown. In regions a and e, a well-dispersed suspension is obtained owing to the higher zeta potential of both components. In region c, both components are dispersed, but a heterocoagulated suspension is obtained owing to their opposite zeta potentials. In the multicomponent systems, either a well-dispersed suspension or a heterocoagulated suspension is used to obtain a homogeneous microstructure. For a dispersed suspension, segregation during colloidal filtration is a common problem owing to differences in the sedimentation rate, but it can be minimized by using a suspension with a high solid content.

pH and zeta potential of two components of A and B, and the dispersion state of both components.
The heterocoagulation method is also applicable to the preparation of porous materials as is schematically shown in Fig. 5 (Tang F. et al., 2003a, b, 2004; Sakka Y. et al., 2005a). This is based on the templating-assisted approach of a core-shell composite, in which monodisperse polymer spheres are used as templates, and ceramic particles act as the target materials. By particle surface modification, well-dispersed suspensions of the polymer and ceramic particles with high opposite charges can be obtained at the same pH. When two suspensions are mixed, an electro-static attractive force is induced between the polymer and ceramic particles, and the polymer particles are uniformly modified by the ceramic particles. After vacuum filtration of the suspension obtained by the heterocoagulation process, the composite is subjected to calcination at a predetermined temperature to remove the spherical polymer particles and loosely sinter the ceramic particles. Through these processes, ordered porous ceramics with regularly arranged pores having the same shape are obtained.
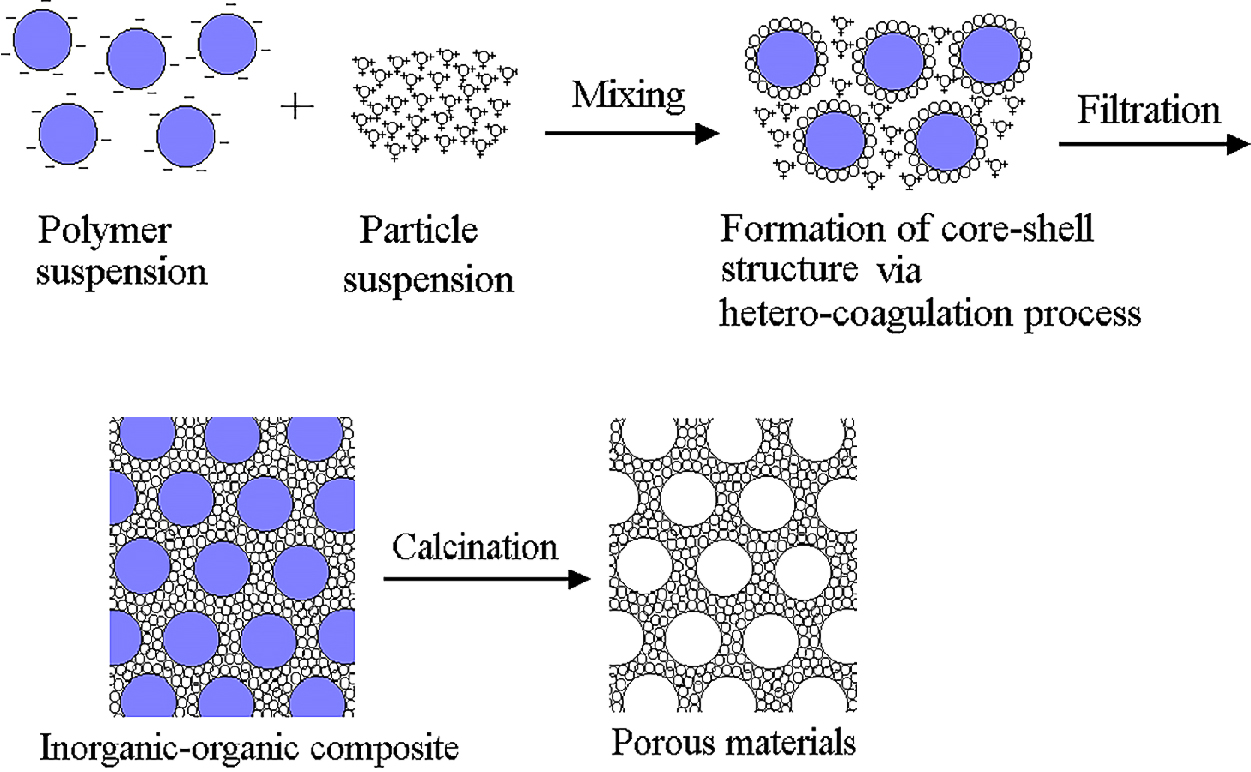
Schematic of fabrication process of ordered porous ceramics. Reprinted with permission from Ref. (Tang et al., 2004). Copyright: (2004) Elsevier B.V.
Fig. 6 shows the relationship between the pH and zeta potential of aqueous suspensions when (1) polymethyl-methacrylate (PMMA) particles 1300 nm in size, (2) titania particles 30 nm in size, and (3) titania and polyethyleneimine (PEI) particles, were added (Tang F. et al., 2003a). Appropriate amount of PEI was added as a polyelectrolyte to the suspension containing titania to add positive charges to the titania particles, which are negative at pH = ~8, similarly to PMMA. Well-dispersed suspensions with PMMA and with titania and PEI were prepared at pH = ~8 and mixed to enable heterocoagulation. Fig. 7 shows a scanning electron microscopy (SEM) image of a fractured surface of porous titania obtained by vacuum casting, calcinating for 4 h at 500 °C, and heating again at 850 °C (Tang F. et al., 2003a). It can be seen that three-dimensional porous titania with a uniform pore size was obtained.

Relationship between pH and zeta potential when PMMA, titania, or titania + PEI is added. Reprinted with permission from Ref. (Tang et al., 2003a). Copyright: (2003) Elsevier B.V.

SEM images of porous titania. High magnification (upper) and low magnification (lower). Reprinted with permission from Ref. (Tang et al., 2003a). Copyright: (2003) Elsevier B.V.
Using this method, porous materials of different systems with different particle sizes may be easily fabricated by changing the type and size of the ceramic and polymer particles (Tang F. et al., 2004; Sakka Y. et al., 2005a).
3.2 Highly conductive carbon nanotube (CNT)- dispersed aluminaCNTs with excellent strength and elastic moduli as well as high chemical, thermal and electrical properties (Iijima S. et al., 1996; Falvo M.R. et al., 1997; Palaci I. et al., 2005) have been considered as an ultimate additive to improve the mechanical and electrical properties of conventional ceramics such as alumina. As a method of increasing its conductivity while maintaining its mechanical properties, large amount of multiwall carbon nanotubes (CNTs) is added to alumina. In fact, however, the ability of CNTs to directly improve the macroscopic mechanical properties of CNTs reinforced ceramics has been questioned and debated mainly owing to CNTs agglomeration, poor CNT/matrix interfacial compatibility, CNTs damage during processing (Estili M. and Sakka Y., 2014a). To this end, it is necessary to prepare a mixed powder in which CNTs and alumina are well dispersed and to fabricate a dense sintered material (Estili M. and Sakka Y., 2014a).
High-purity CNT (Bussan Nanotech), synthesized by a catalytic-CVD process and graphitized at about 2600 °C, was prepared and functionalized as follows. The CNT powder was treated with a mixture of H2SO4 and HNO3 with a volume ratio of 3:1 at 110 °C for 20 min. The oxidative agent produced by the sulfonitric mixture was found that oxidation starts with the C-C and C-H bonds generating different oxidized groups from alcohol to carboxylic acid, following a sequential oxidation (Gomez S. et al., 2017). The mixtures were cooled with water and washed up to pH = 7. The hydrophilic CNT powder obtained was dispersed in water by ultrasonic irradiation at pH = 4.4, resulting in the surfactant-free and COOH-terminated CNT aqueous suspension.
Suspensions of the hydrophilically treated CNT powder and alumina powder were separately prepared (Estili M. and Kawasaki A., 2008; Estili M. et al., 2008, 2012, 2013). Considering the different surface potentials of CNTs and alumina under acidic conditions, the suspensions were mixed at pH = 4.4 and subjected to heterocoagulation to obtain a well-dispersed mixed powder (Fig. 8). By SPS of the CNT–alumina mixed powder (Fig. 9, upper) at 1300 °C, a dense nanocomposite of 20 vol% well-dispersed CNT dispersed alumina (Fig. 9, lower) was obtained. This sintered material exhibited an electrical conductivity of 5000 S/m, which is the highest reported for an alumina-based material (Estili M. et al., 2012, 2013). The bending strength and fracture toughness of the obtained sintered material with different amounts of CNTs increased up to a CNTconcentration of 10 vol%. It is seen in Table 2 that the bending strength and fracture toughness obtained with the addition of 20 vol% CNTs were similar to those without the addition of CNTs (Estili M. et al., 2012).
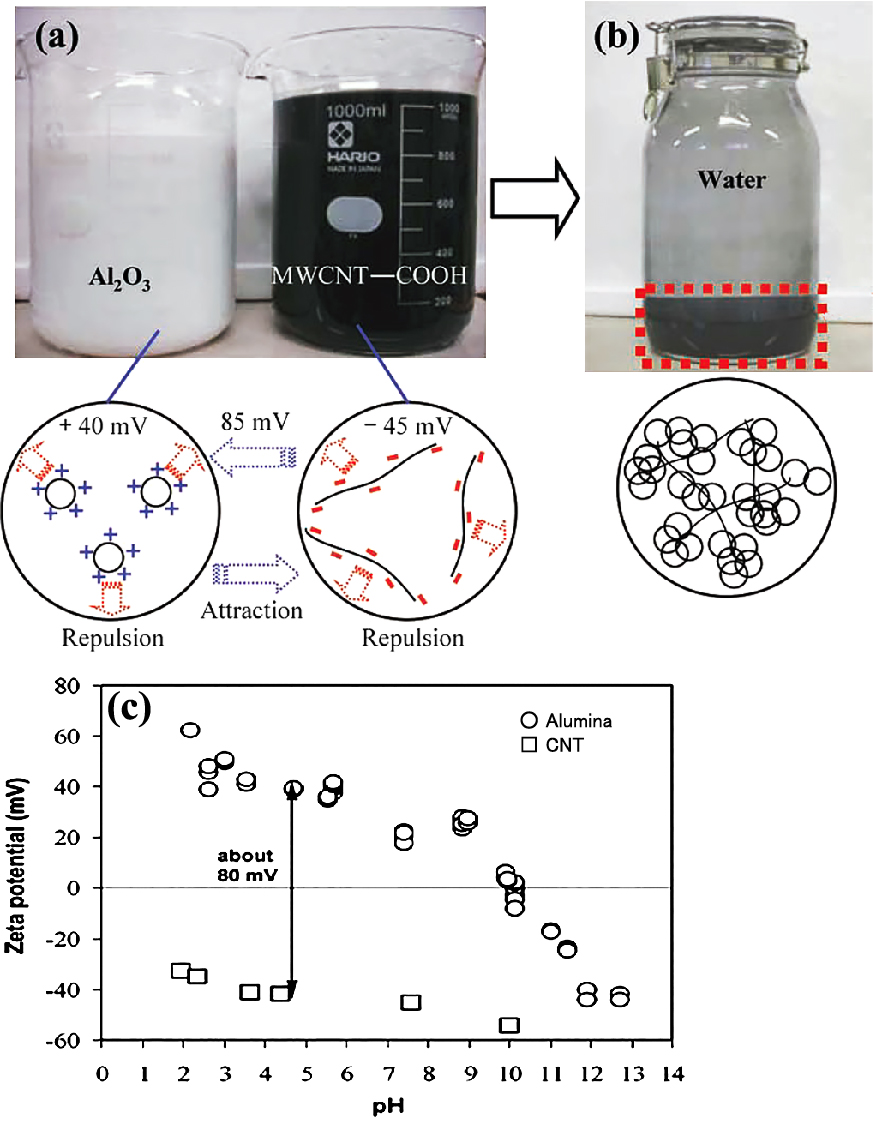
Suspensions of alumina, CNT powder after hydrophillic treatment, and heterocoagulated suspension. Reprinted with permission from Ref. (Estili M., 2012). Copyright: (2012) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
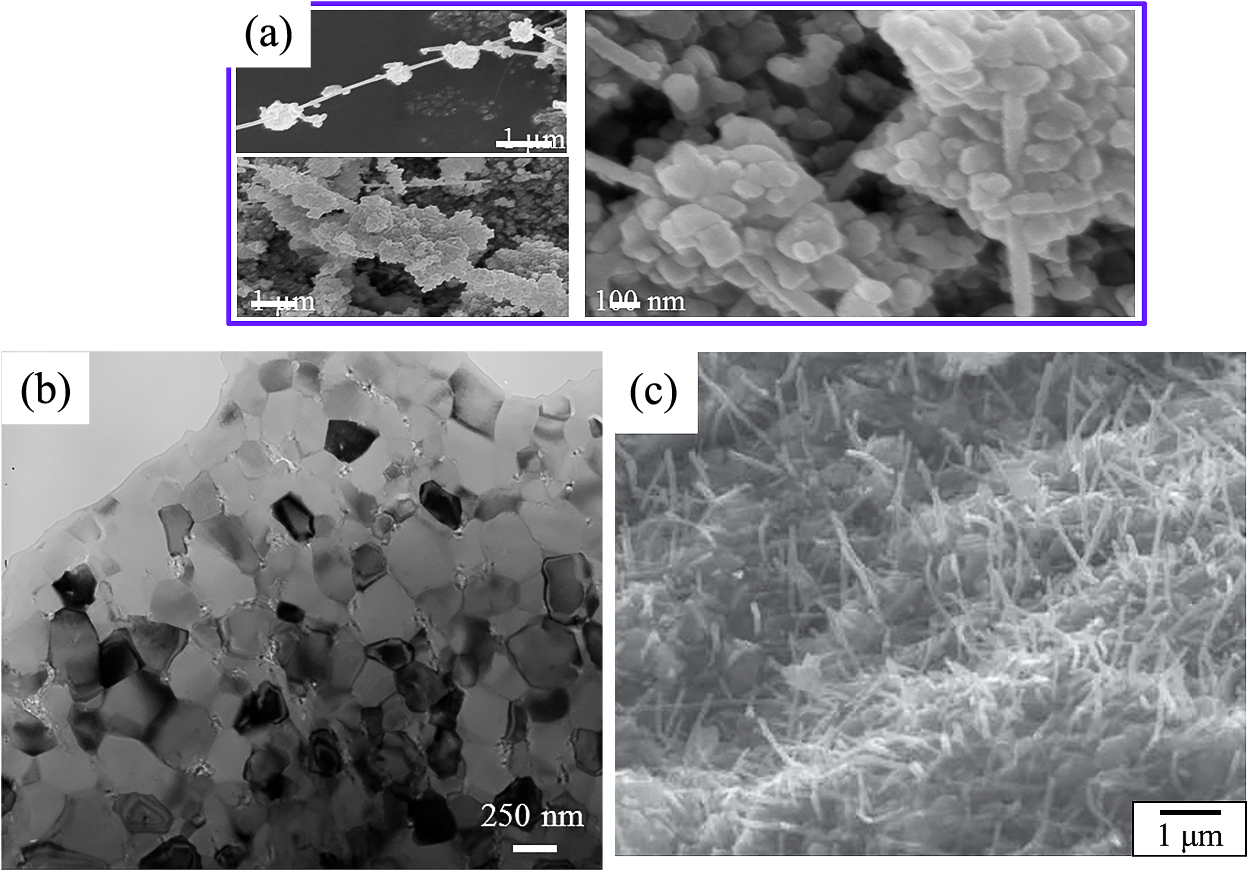
SEM photos of the heterocoagulated mixed powders (a), and TEM photo (b) and fractured SEM photo (c) of the dense nanocomposite of 20 vol%-CNT dispersed alumina after SPS at 1300 °C. Reprinted with permission from Ref. (Estili M., 2012). Copyright: (2012) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
| SPSed bulks | Density (g cm−3) | Strain to failure (%) | K1C(MPa m0.5) Three-point bending (SENB method) | Flexural strength (MPa) Four-point bending |
|---|---|---|---|---|
| α-Al2O3 (reference material) | 3.960 (100%) | 0.105 | 4.41 | 395.82 |
| 2.0 vol% hybrid | 3.901 (99.44%) | 0.11 (+8.2%) | 5.78 (+31%) | 413.46 (+4.5%) |
| 10.0 vol% hybrid | 3.733 (98.91%) | 0.19 (+81%) | 6.71 (+52.2%) | 483.19 (+22%) |
| 20.0 vol% hybrid | 3.544 (98.8%) | 0.21 (+98%) | 4.62 (+4.7%) | 403.72 (+2%) |
This method has been attracting interest as a fabrication method for high-strength, high-conductivity oxide and non-oxide ceramics (Estili M. and Sakka Y., 2014a; Wu W.W. et al., 2017). This method is also applicable to the graphene dispersed ceramics (Estili M. et al., 2014b).
The rotation of particles in a magnetic field is caused by the generation of a magnetic torque due to magnetic anisotropy. If the crystal structure is asymmetrical, such as tetragonal or hexagonal, the magnetic susceptibility depends on the direction of the crystal axis and shows anisotropy. On the basis of the interaction between the anisotropy and the magnetic field, the magnetic torque given by Eq. (2) induces the rotation of particles (Suzuki T.S. et al., 2001; Sakka Y. and Suzuki T.S., 2005b).
| (2) |
Here, T is the magnetic torque, μ0 is the magnetic the permeability of vacuum, Δχ is the anisotropy of magnetic susceptibility, V is the volume of particles, B is the magnetic flux density, and θ is the angle between the easy axis of magnetization and the direction of the applied magnetic field. The magnetic torque is usually regarded as negligible because the magnetic susceptibilities of para-magnetic and diamagnetic materials are extremely small. However, with recent advances in the technology of superconductivity, strong magnetic fields exceeding 10 T are now more easily obtained without supplying liquid He, enabling orientation using magnetic torque. Our research group has demonstrated that it is possible to control the crystal orientation of alumina, titania, aluminum nitride, silicon carbide, zinc oxide, silicon nitride, HAP, and piezoelectric ceramics, all of which are diamagnetic and have extremely small magnetic susceptibility, by colloid processing in a strong magnetic field (Sakka Y. and Suzuki T.S., 2005b; Suzuki T.S., et al., 2006a). The uniaxial orientation of alumina and titania is possible by the application of a static magnetic field because their magnetic susceptibilities along the c-axis are higher than those along the a, b-axis (Sakka Y. and Suzuki T.S., 2005b; Suzuki T.S. et al., 2006b). In contrast, for aluminum nitride, silicon carbide, zinc oxide, silicon nitride, and HAP, only a c-plane-oriented material is obtained when a static magnetic field is applied because their magnetic susceptibilities along the a, b-axis are higher than those along the c-axis. In this case, uniaxially oriented materials are fabricated using a rotating magnetic field (Tanaka S. et al., 2006; Suzuki T.S., et al., 2009, 2010; Zhu X. and Sakka Y., 2008a; Zhu X. et al., 2010, 2014).
Fig. 10 compares the Lotgering orientation factor (Lotgering F.K., 1959), grain size, and relative density of heavily agglomerated HAP after ultrasonic irradiation (R-HAP) and redispersed HAP after bead milling treatment (B-HAP) after sintering at fixed temperatures. The higher degree of orientation for the B-HAP is mainly due to the deagglomeration by the milling procedure. It is well known that the slip casting of a well-dispersed suspension yields a dense green body with a narrow pore size distribution, which results in a dense and fine-grained micro-structure characterized by low-temperature sintering. This colloidal processing technique is also useful for obtaining highly oriented ceramics.
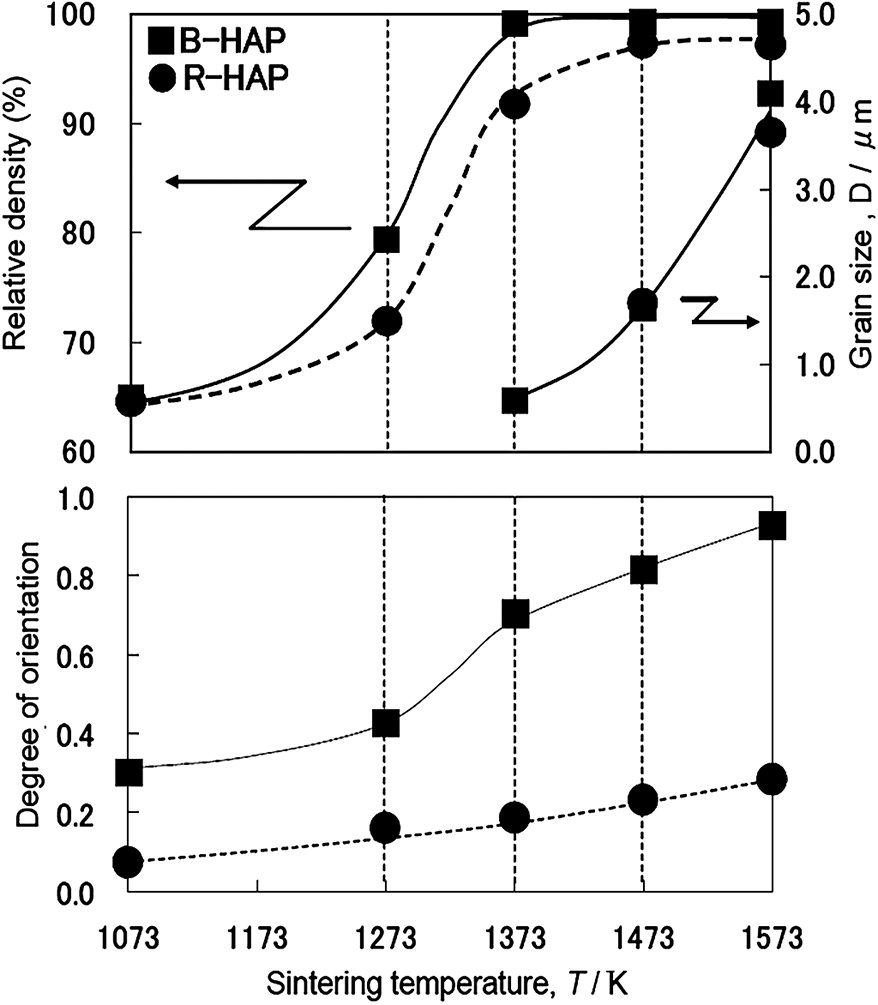
Temperature dependences of relative density and the grain size (upper), and the Lotgering orientation factor (lower) of heavily agglomerated R-HAP as ultrasonic irradiation and de-agglomerated B-HAP after bead milling (shown in Fig. 2).
It can be seen that the orientation factor is promoted by sintering at higher temperatures. The magnetic energy is proportional to the volume of the particle, the square of the magnetic field, and the difference between the crystal susceptibility of a, b and that of c. The orientation factor of HAP is basically determined by the particle size and the magnetic field when each particle is well dispersed. However, owing to thermal fluctuation, each particle tends to align in a specific direction with some distribution of the angle. Therefore, the orientation factor is not so large initially. Then the orientation factor increases with grain growth, where the oriented particles act as a template for the oriented grain growth. These features are confirmed by in situ observation (Hirota N. et al., 2008; Terada N. et al., 2008).
To obtain oriented materials with weak magnetic susceptibility, the following conditions are necessary (Sakka Y. and Suzuki T.S., 2005): (1) the particles should be single-crystal and well dispersed, (2) the crystal structure should be noncubic to yield an anisotropic magnetic susceptibility, (3) the magnetic energy should be larger than the thermal motion energy, (4) the viscosity of the suspension should be sufficiently low for the particles to be rotated with a low energy, and (5) grain growth is necessary to obtain a highly oriented structure, particularly when spherical particles are used. For colloidal processing, slip casting, gel-casting, and EPD have been conducted as will be shown later.
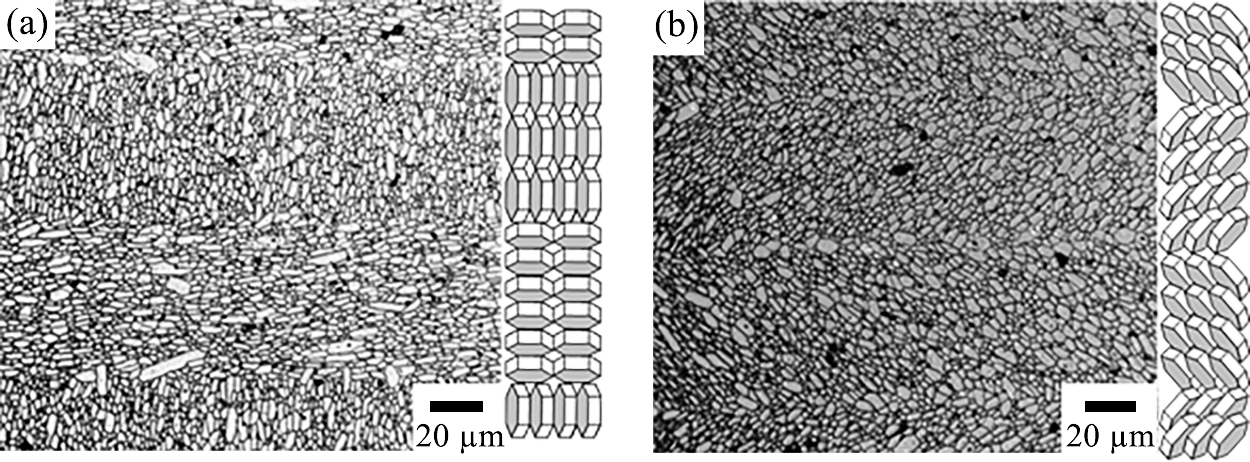
As shown in the previous session, when the magnetic susceptibility along the a, b-axis is higher than that along the c-axis, uniaxially oriented materials can be fabricated using a rotating magnetic field. As an example of the merit of using a rotating magnetic field, the processing of textured β-Si3N4 is discussed (Zhu X.W. et al., 2006, 2008, 2010). α-Si3N4 (Ube Industry’s SN-E10) was used as a raw powder, and 5 mol% Y2O3 and 5 mol% Al2O3 were added as sintering aids. As a seed, 5 mol% β-Si3N4 was added. Ethanol was used as a solvent, 1 wt% PEI was used as a dispersant, and a 30 vol% slurry was prepared. After slip casting under 12 T followed by consolidation, drying, cold isostatic pressing (392 MPa), and calcination (500 °C for 2 h in air), sintering was conducted in a graphite resistance furnace at 1800 °C in 0.2 MPa N2.
As shown in Table 3, a static magnetic field leads to a large decrease in the intensity ratio between the (200) and (101) planes from the top surface to the side surface, indicating the orientation of the a, b-axis (Zhu X.W., et al., 2008b, 2010). On the other hand, the rotating magnetic field allows the intensity between the (002) and (200) planes to become zero on the side surface, indicating the successful orientation of the c-axis. Clearly, the addition of the β-Si3N4 seed leads to the substantially higher orientation of β-Si3N4 crystals in green bodies in both cases, as indicated by the larger intensity ratios on the top surfaces. Fig. 11 schematically shows orientation mechanism of static and rotating magnetic fields. Fig. 12 shows the microstructures of β-Si3N4 slip-cast in a static magnetic field of 12 T (left) and β-Si3N4 consolidated in a rotating magnetic field (right). Elongated β-Si3N4 grains were observed in random directions on an oriented c-plane in a static magnetic field. In contrast, elongated β-Si3N4 grains were oriented uniaxially along the c-axis in a rotating magnetic field.
| Sample | Tested surface# | Texture in green body | Texture in sintered body | ||||
|---|---|---|---|---|---|---|---|
| XRD Intensity ratio | Lotgering orientation factor (f*) | ||||||
| SMF | RMF | SMF | RMF | ||||
| I(200)/I(101) | I(002)/I(200) | 15 min | 6 h | 1 h | 3 h | ||
| Non-seeded | TS (⊥ B) | 2.90 | ≈0.5 | 0.68 | 0.88 | 0.17 | 0.24 |
| SS (//B) | 0.75 | ≈0 | |||||
| Seeded | TS (//B) | 12.4 | 5.07 | 0.86 | 0.97 | 0.49 | 0.51 |
| SS (⊥ B) | 0.89 | ≈0 | |||||
(JCPDS card), P = ∑I(hk0)/∑I(hkl) (sample); for RMF, P0 = ∑I0(00l)/∑I0(hkl), P = ∑I(00l)/∑I(hkl).

Schematic of orientation mechanisms of β-Si3N4 crystals during slip casting in (a) static and (b) rotating magnetic fields.
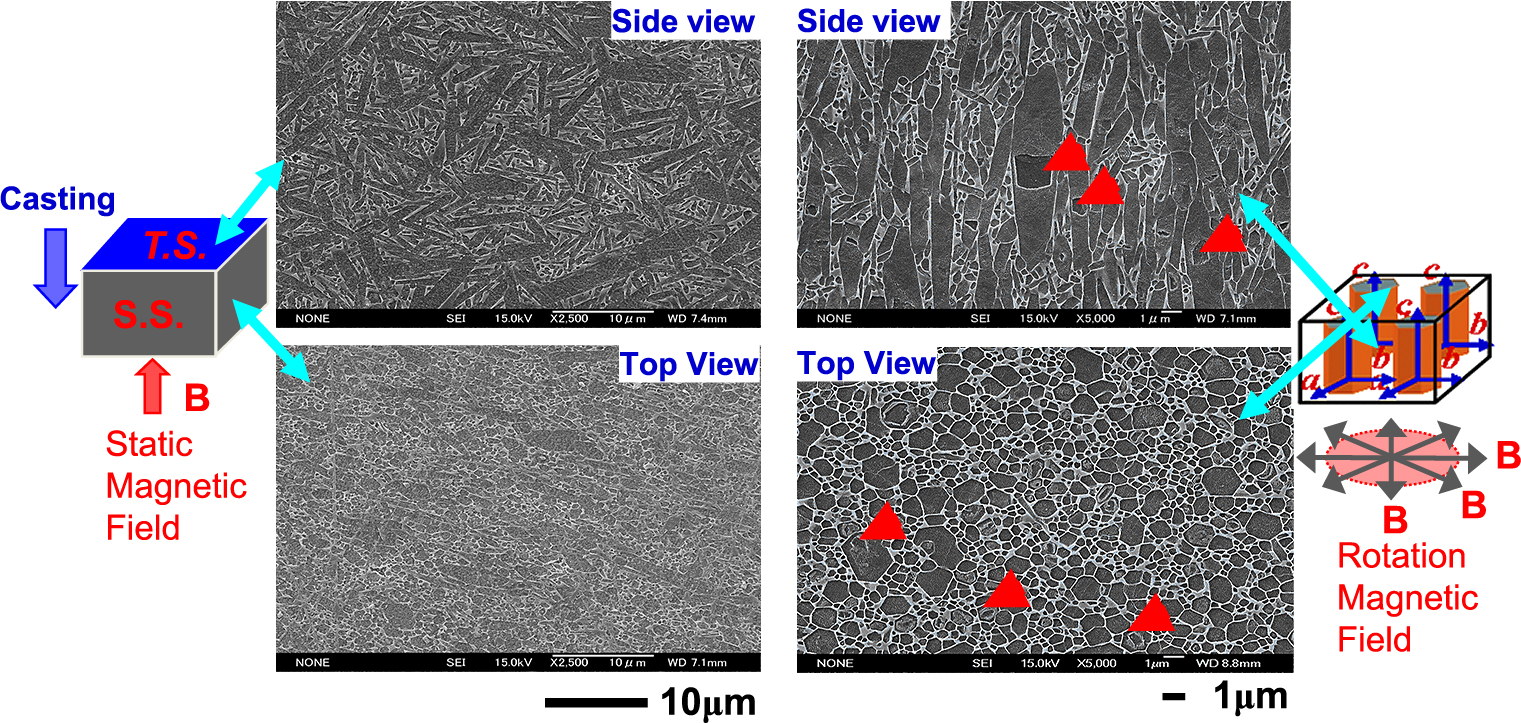
SEM microstructures of β-Si3N4 slip-cast in a static magnetic field of 12 T (left) and β-Si3N4 consolidated in a rotating magnetic field (right) after sintering at 1800 °C for 5 h. Reprinted with permission from Ref. (Zhu et al., 2008b). Copyright: (2008) Trans Tech. Pub. LTD.
Using Y2O3 and MgSiN2 powders as sintering aids, the fabrication of β-Si3N4 with a high thermal conductivity of 176 Wm−1K−1 along the c-axis was successful (Zhu X.W. et al., 2014). When silicon nitride thus obtained is used as a heat-radiating substrate, the c-axis can correspond to the thickness direction. Thus, heat-sink substrates made of β-Si3N4 are expected to be used in power electronics.
To control the structure of high-functionality materials, biaxial or triaxial orientations are desired rather than a uniaxial orientation.
Bi3TiNbO9 planar particles with a biaxial orientation can be obtained by employing gravity and applying a magnetic field (Keskinbora K. et al., 2010; Suzuki T.S. et al., 2013; Gao Z.P. et al., 2015). For platelet particles with micron-order sizes, the largest energy in the dispersion state is the gravitational energy. Therefore, platelet particles are first oriented parallel to the bottom surface, and then they are oriented biaxially by applying a magnetic field. In this case, the lattice constants a and b are similar (Suzuki T.S. et al., 2013). To obtain a triaxially oriented ceramics, platelet Bi4Ti4O15 particles, for which the lattice constants a, b, and c are different in the orthorhombic structure, were used and a two-step procedure involving the application of a magnetic field from two directions sequentially were employed (Suzuki T.S. et al., 2016). In addition, triaxially oriented Y2Ba4Cu7Oy, which is para-magnetic at room temperature, was obtained in an epoxy resin by changing the rate of rotation of a magnetic field at a modulated rate (modulated rotating magnetic field) (Fukushima T. et al., 2008).
5.2 Nacre-like ceramicsIn general, the strength and toughness of ceramics are very difficult to enhance simultaneously; these two factors usually show opposite tendencies. The intrinsic brittleness of ceramics also limits their wide application. The keys to improve material characteristics by controlling the micro-structure can be found in nature (Lin A.Y.M. et al., 2006; Munch E. et al., 2008). Through the process of evolution over millions of years, plants and animals have selected the optimal structures to survive in harsh environments. For example, oriented tabular calcium carbonate layers with a thickness of ~100 nm are laminated in nacre. At the interfaces of the layers, a layer containing several percent of protein membranes is inserted to form an oriented laminated structure (hierarchical structure) in Fig. 13(a) (Lee S.W. et al., 2008; Meyers M.A. et al., 2008). Compared with sintered calcium carbonate, the hierarchical structure has much higher strength and toughness (fracture toughness). To improve both the strength and toughness simultaneously, the microstructure of the material must have (1) weak grain boundary interfaces and (2) rodlike particles and a tabular structure (Lawn B. R. et al., 1994; Tang Z. et al., 2003). Using these requirements as guidelines, Mn+1AXn (MAX; M, transition metal; A, group A element; X, C or N) phase ceramics have attracted attention as nanolaminates, where ideally n = 1–3. MAX has a layered structure (Fig. 14) (Barsoum M.W. and EI-Raghy T., 2001; Wang X.H. and Zhou Y.C., 2010; Sun Z.M., 2011).

Microstructures of (a) nacre-like layer and (b) textured Nb4AlC3 ceramic. Reprinted with permission from Ref. (Hu et al., 2011a). Copyright: (2011) Elsevier B.V.
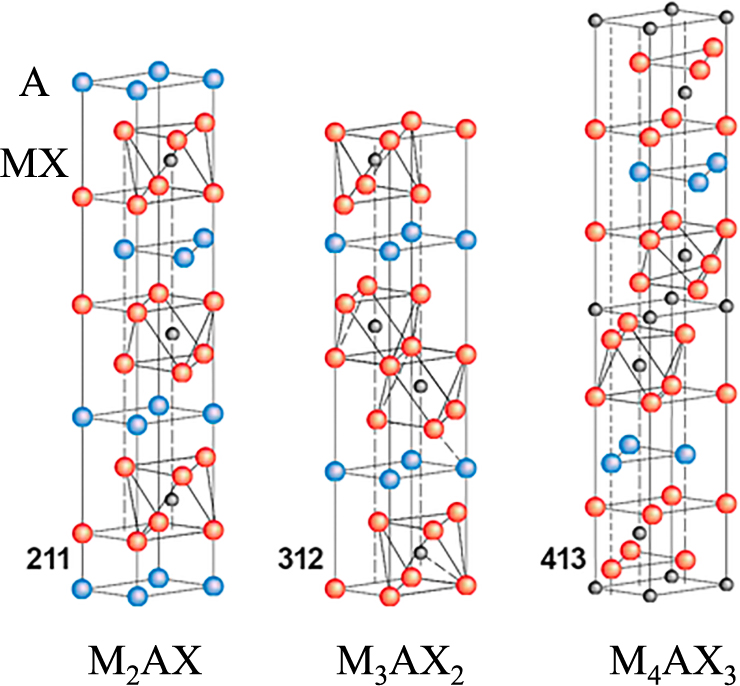
Layered structures of three types of MAX phase ceramics.
As an example, the results of the microstructure and mechanical properties for the MAX phase ceramic Nb4AlC3 are shown (Hu C. et al., 2011a, b, c). The weak bonding between Al atom layers, and Nb and C atom layers contributes to the easy formation of dislocations and their slipping, which induce the development of kink bands in the grains exhibiting “quasi-plastic” behavior. To align Nb4AlC3 nanoparticles on a layer, a suspension of Nb4AlC3 particles is slip cast in a strong magnetic field and subjected to SPS to obtain a dense compact. From SEM and transmission electron microscopy (TEM) images, the obtained structure has a nacre-like microstructure with layer stacking from the nanoscale to the milliscale shown in Fig. 13(b). Undoubtedly, the microstructure design explains the mechanical responses. Single-edge notched bending (SENB) samples tested along the c-axis direction exhibited the zigzag fracture mode. The zigzag fracture surface corresponds to a high surface energy transformed from the mechanical energy. Additionally, the investigation of the microscale zigzag fracture surface revealed pull-out grains distributed on the entire surface, which means that the toughening mechanisms may involve crack deflection, which increases the surface energy, and crack bridging, which lowers the stress intensity factor at the crack tip (Dericioglu A.F. and Kagawa Y., 2002)
Fig. 15 shows a diagram of the flexural strength and fracture toughness of the textured Nb4AlC3 ceramic in comparison with those of other advanced ceramics and other textured MAX phase ceramics (Zhang H.B. et al., 2015). Here the oxide phase is introduced during the powder processing. Textured Nb4AlC3 ceramics have the highest bending strength and fracture toughness. The textured MAX phase ceramics are also expected to show anisotropic properties such as electric and thermal conductivities, oxidation resistance, and tribological properties (Xu L.D. et al., 2017). In addition, the processing of MAX phase ceramics is straightforward (Mishra M. et al., 2012a, b; Zhou T.L. et al., 2014; Sato K. et al., 2014a, b; Idzkowska A. et al., 2015). Therefore, structural components with a complicated shape can be easily formed, which is expected to lead to the development and design of high-performance layered ceramic materials.

Relationship between bending strength and fracture toughness of textured MAX phase ceramics and other ceramics, where oxide dispersed MAX phase ceramics was prepared by oxidation of MAX phase powder during powder processing. This figure is revised and added newly obtained data of the Ref. (Hu C. et al., 2011a).
As shown in session 2, during the EPD in which particles themselves move, the deposition rate does not depend on the particle size and is extremely fast. Based on this point, EPD is a suitable method for consolidating nanoparticles and EPD has received a significant amount of attention for fabricating highly structured controlled ceramics resulting in advanced ceramics. As compared with tape casting, EPD is also suitable for the production of thickness-controlled laminates with good adherence between the layers (Uchikoshi T. et al., 2002). Therefore, recently EPD has recently been applied in many fields.
We have demonstrated that EPD in a high magnetic field is an excellent method to fabricate thick crystalline textured ceramic bodies (Uchikoshi T. et al, 2003, 2004). Fig. 16 shows a schematic of the experimental setup used for EPD in a strong magnetic field. By changing the angle between the magnetic field and the electric field (ϕB–E) during the superposed application of the two fields, the crystal orientation with respect to the substrate is controlled. In addition, by changing ϕB–E during EPD at predetermined intervals, layers with different crystal orientations can be deposited.
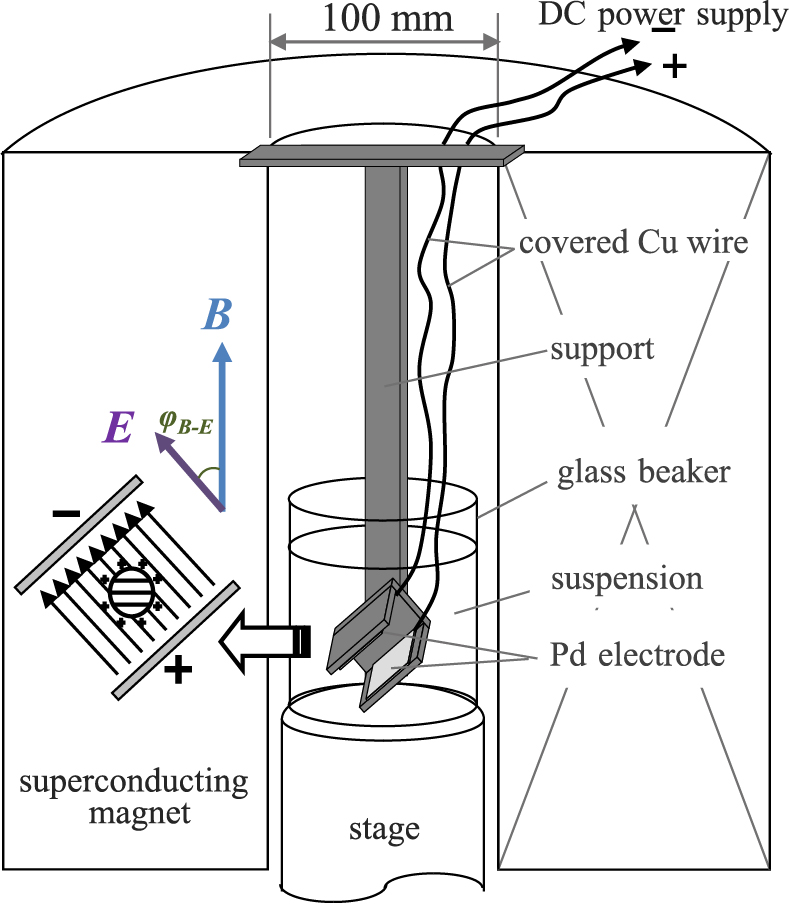
Schematic of EPD in strong magnetic field.
Fig. 17 shows an example of a crystalline textured alumina/alumina laminated composite deposited by alternately applying magnetic fields with (a) ϕB–E = 0° and 90° and (b) ϕB–E = 45° and −45°. It was possible to align the crystal orientations by controlling ϕB–E, regardless of the crystal orientation of the underlying layer, even when the oriented layers were laminated (Uchikoshi T. et al, 2003, 2004; Suzuki T.S. et al., 2006b).
Crystalline textured alumina/alumina laminated composites deposited by alternately applying magnetic fields with (a) ϕB–E = 0° and 90° and (b) ϕB–E = 45° and −45°. Reprinted with permission from Ref. (Suzuki et al., 2006b). Copyright: (2006) NIMS & Elsevier B.V.
The orientation and laminating technology is effective not only for controlling the mechanical properties but also for improving the functions and reliability of thermoelectric elements, photoelectrodes, ion conductors, piezoelectrics, and dielectrics (Horii S. et al., 2007; Okamoto T. et al., 2006; Kawakita M. et al., 2009; Yamada H. et al., 2013; Zhang C.N. et al., 2014; Miwa Y. et al., 2015; Matsuda M. et al., 2016).
Colloidal processing has been attracting attention as a consolidation process utilizing the advantages of fine particles. Controlling the dispersion of fine particles will enable the fabrication of dense sintered materials, porous ceramics and CNTs-ceramic nanocomposites. In addition, applying electric and magnetic fields externally during consolidation is expected to enable the advanced control of the microstructure. In this review, examples of the fabrication of nacre-like ceramics with textured MAX phase ceramics and laminated composites were introduced. Various material properties, such as corrosion resistance, wear resistance, thermal conductivity, electric conductivity, piezoelectric properties, and transparency, depend on the crystal orientation. Therefore, the method outlined in this review is applicable to all ceramics except those with cubic crystals. In future studies, the characteristic structures and related properties will be analysed to optimize the material structure, which is expected to lead to the development of advanced functional ceramics.
This study was supported by many colleagues and post-doctoral researchers at the National Institute of Materials Science, and students at the University of Tsukuba, Tokyo University of Science, Hosei University, and Shibaura Institute of Technology. In particular, I would like to express my sincere thanks to Dr. T. Suzuki (senior researcher), Dr. T. Uchikoshi (group leader), Dr. F.Q. Tan, Dr. S. Grasso, Dr. C. F. Hu and Dr. K. Sato, Dr. X.W. Zhu, and Dr. M. Estille for the support in studies on magnetic field orientation, the EPD process, ordered porous materials, the SPS process, high-intensity MAX phase ceramics, the advances in silicon nitride, and the fabrication of CNT nanocomposites, respectively.
Yoshio Sakka
Dr. Yoshio Sakka is a Senior Scientist and an advisor of Graduate Program Office at NIMS, Japan. He received his B.E. in 1978, M.E in 1980, and PhD in 1983 from Kyushu University. After receiving his PhD he joined the National Research Institute for Metals (present NIMS). He is the author or coauthor of 19 books, above 650 original referee’s papers, above 100 review papers, and above 80 patents (including application). By the above establishment, he received many awards, such as Academy member of World Academy of Ceramics (2009), Richard Brook Award from European Ceramic Society (2011), KONA Award (2014) .