2019 年 36 巻 p. 129-144
2019 年 36 巻 p. 129-144
Significant progress has been made over the last half-century in delivering therapeutics by the pulmonary route. Inhaled therapeutics are administered to humans using metered-dose inhalers, nebulizers, or dry powder inhalers, and each device requires a different formulation strategy for the therapeutic to be successfully delivered into the lung. In recent years, there has been a shift to the use of dry powder inhalers due to advantages in the consistency of the dose delivered, ease of administration, and formulation stability. Numerous preclinical studies, involving small and large animals, have evaluated dry powder drugs, vaccines, and immunotherapeutics delivered by the pulmonary route. These studies used different dry powder delivery devices including nose-only, whole-body, and intratracheal administration systems, each of which works with different aerosolization mechanisms. Unfortunately, these delivery platforms usually lead to variable powder deposition in the respiratory tract of animals. In this review, we will discuss obstacles and variables that affect successful pulmonary delivery and uniform powder deposition in the respiratory tract, such as the type of delivery device, dry powder formulation, and the animal model used. We will conclude by outlining factors that enhance the reproducible deposition of dry powders in the respiratory tract of preclinical animal models and identifying knowledge and technology gaps within the field. We will also outline the important factors necessary for successful translation of studies performed in preclinical models to humans.
Inhaled therapies and medicines date back to 2000 B.C. in India, where datura roots were smoked for their broncho-dilating properties (Anderson, 2012). The ancient Egyptians, the Greeks, and Native cultures in Central and South America all used different forms of inhalable medicines (Anderson, 2012). Even inhaled insulin, a therapy considered relatively recent, was first explored in 1925 by a German scientist named Gansslen (Ghosh and Collier, 2007; Patton and Byron, 2007).
However, the development of dry powders for inhalation by the pharmaceutical industry is a more recent development, with the first commercially available dry powder inhaler (DPI) patented in 1949 (Fields, 1949; Stein and Thiel, 2017). Dry powder therapeutics continue to be a popular pulmonary therapeutic option for patients because of the consistency in the lung dosing, ease of administration, short delivery time, and the excellent stability that is inherent to these formulations (Telko and Hickey, 2005; Vehring, 2008; Sou et al., 2011; Kunda et al., 2016). Since 1987, the number of commercially available DPIs has dramatically increased, and preclinical drug development using dry powders has grown rapidly (Stein and Thiel, 2017).
It is interesting, then, that pulmonary delivery of medicines often meets with resistance and pushback from the general medical community, and that the transition of successful preclinical drug and therapeutic candidates to the clinical trial stage, and finally to the market, has been difficult. A strong argument can be made that this is the result of a lack of the basic foundational tools and regulations which are required to make this transition smoother. This article will review the benefits of pulmonary drug administration, specifically dry powder aerosols, and follow up with an examination of several weak points of preclinical pulmonary delivery of dry powders and how these might be restricting successful translation to humans. Furthermore, the authors will propose areas in need of improvement and speculate on the directions that future dry powder pulmonary delivery in preclinical models needs to take.
The human lung is an excellent platform to deliver drugs both locally and systemically. The large surface area, rapid absorption due to dense vasculature, minimal enzymatic activity (no hepatic first pass), and thin alveolar epithelium (0.1 μm to 0.2 μm) make the lungs an attractive, non-invasive site for delivering drugs (Patil and Sarasija, 2012).
The lungs are a highly structured tissue which resembles an inverted tree. In humans, the primary bronchi divide into the left and right lung, and each lobe further divides into three lobes on the right side and two lobes on the left (Kunda et al., 2013). Each lung branches into at least 23 generations with the first 16 generations made up of bronchi and bronchioles, and by the 17th generation alveoli begin to appear on the respiratory walls (Weibel, 1963). Each branch always divides into two smaller branches or bronchioles (dichotomous branching). By the 20th generation, alveolar ducts begin to appear and the entire lung wall is made up of alveoli. Toward the last generations, the alveolar ducts end in alveolar sacs (Effros, 2006; Kunda et al., 2013) (Fig. 1). Together, these lung branches result in a large surface area of approximately 140 square meters (Fernandes and Vanbever, 2009).

Human lung with generations. (Adapted from (Kleinstreuer, Zhang and Donohue, 2008; Kunda et al., 2013))
The physical, chemical, and physiological factors inherent to the inhaled particles of a particular drug affect the behavior of and ultimately the deposition of the drug in the lungs (Scheuch et al., 2006; Demoly et al., 2014). Proper consideration of the above factors allows for the development of an inhalation product that will deposit reproducible amounts of the drug into the lungs across patient populations. In addition to these factors, barriers to drug deposition and absorption presented by the diseased lung anatomy should be taken into consideration. Barriers such as mucus thickness, alveolar lining fluid, uptake by macrophages, and proteolytic degradation must be adequately incorporated in the design of a suitable formulation for inhalation (Scheuch et al., 2006).
2.2 Formulation and delivery of inhalation productsThe formulation of a drug intended for pulmonary delivery together with an inhaler device constitutes an inhalation product (Hou et al., 2015). The compatibility between the inhalation device and the formulation, comprised of the active pharmaceutical ingredient (API) and the excipients, is essential to efficiently deliver the API to the target site. An ideal inhalation product must be easily operated, uncomplicated, handy, should deliver reproducible doses irrespective of temperature, humidity, and patient inspiratory flow rate (Bäckman et al., 2014).
An optimized formulation in a suitable inhaler is crucial for a successful inhalation product. The physico-chemical properties of the formulation such as particle size, morphology, size distribution, particle growth, and solid-state characteristics influence the deposition profile of the API in the lungs (Shaji and Shaikh, 2016). A review by Shaji and Shaikh discussed in detail the characterization techniques used to evaluate various aerosol drug delivery systems (Shaji and Shaikh, 2016). Currently, there are three main pulmonary drug delivery devices marketed: a) nebulizers, b) pressurized metered-dose inhalers (pMDIs), and c) dry powder inhalers (DPIs). The device employed for an inhalation product depends on the properties of the drug substance, choice of excipients, and patient compatibility. In addition, other factors that affect the choice of inhaler include device availability, clinical setting (inpatient, outpatient, emergency department, etc.), patient age, correct usage, device cost, administration duration, and patient convenience (especially during long-term use) (Dolovich et al., 2005).
Furthermore, the choice of drug state (aqueous vs solid), excipients, and device determine the inhalation product (nebulizer vs pMDI vs DPI). For example, albuterol sulfate is available as: a) Ventolin HFA (inhalation product), wherein the drug is a suspension (API in HFA 134a) and device is a pMDI, and b) ProAir Respiclick dry powder, wherein the drug is a dry powder (blend of API and lactose monohydrate) and device is a DPI.
2.3 Pulmonary drug delivery marketOver the last few decades, inhaled product development has seen huge success with hundreds of medications making it to the market (Forbes et al., 2011). Medications providing relief to local lung diseases such as asthma and chronic obstructive pulmonary disorder (COPD) are the major drivers for the inhalation market (Hickey, 2013). Inhalation product development, against cystic fibrosis (CF) (Garcia-Contreras and Hickey, 2003), chemotherapeutics, and macromolecules against lung cancer (Storti et al., 2015) have seen increased attention and are contributing to the continued interest in the development of new aerosol-based therapies. Furthermore, the ability to achieve rapid absorption of drugs from the lungs into the systemic circulation has initiated efforts to develop inhalable macromolecules. New macromolecule dry powder formulations are being designed for many diseases including diabetes, lung cancer, and alpha 1 antitrypsin deficiency. Most of the formulation testing in preclinical models begin with a liquid form of the macromolecule and subsequently transitions toward a dry powder formulation, probably due to the ease of delivery of liquid formulations in animals. However, this linear development process does not lead to novel dry powder inhalation products and the translational success of de novo dry powder products from preclinical studies to the clinic has been abysmal. The problems associated with this development process will be discussed later in detail in the “Implementation of lessons learned from humans to animals” section.
Table 1 lists a few of the different inhalation products that have been approved over the last few decades. The table shows the expansion of the inhalation market from the 1990’s to date with pMDIs and nebulizers occupying the majority of the market compared to DPIs. Moreover, availability of more than one inhaler type per drug by different manufacturers shows the competition in the inhalation market. Further, with recent advancements in dry powder formulation and inhaler technology, manufacturers have been developing DPI products for APIs currently available as pMDI/nebulizer products (shown as greyed area in the table). In 2015, MDIs accounted for 67 % of inhalation market; however, DPIs are forecasted to report an exceptional compound annual growth rate (CAGR) of 16.9 % over the next few years (Grand View Research, 2016). This shift toward developing DPI products is due to an increase in the physical and chemical stability of compounds formulated into solid-state; a DPI also simplifies the inhalation maneuver required by the patient compared to the other available inhaler technologies.
| Indication | API | Device | Product | Manufacturer | Approval Date |
|---|---|---|---|---|---|
| Asthma and COPD | Albuterol sulfate | pMDI | Proventil HFA | Merck | 1999 |
| pMDI | Ventolin HFA | GlaxoSmithKline | 2001 | ||
| Nebulizer | AccuNeb | Mylan | 2001 | ||
| pMDI | ProAir HFA | Teva | 2004 | ||
| DPI | ProAir Respiclick | Teva | 2015 | ||
| Budesonide | DPI | Pulmicort Flexhaler | AstraZeneca | 1998 | |
| DPI | Pulmicort Turbuhaler | AstraZeneca | 1998 | ||
| Nebulizer | Pulmicort Respules | AstraZeneca | 2000 | ||
| Beclomethasone dipropionate + Formoterol fumarate | pMDI | Fostair | Chiesi | 2007 | |
| DPI | Fostair Nexthaler | Chiesi | 2014 | ||
| Fluticasone furoate + vilanterol | DPI | Breo Ellipta | GlaxoSmithKline | 2013 | |
| Cystic fibrosis | Tobramycin | Nebulizer | Tobi | Novartis | 1997 |
| Nebulizer | Bethkis | Chiesi | 2012 | ||
| DPI | TOBI Podhaler | Novartis | 2013 | ||
| Itraconazole | DPI | PUR1900 | Pulmatrix | Nonclinical Development | |
| Diabetes | Insulin | Soft-mist | Dance-501 | Dance Biopharm Inc | In Development |
| DPI | Afrezza | Mannkind/Sanofi Aventis | 2014 | ||
| Idiopathic pulmonary fibrosis | A kinase inhibitor | DPI | PUR1800 | Pulmatrix | Clinical Trial Phase II |
| Pulmonary arterial hypertension | Treprostinil | Nebulizer | Tyvaso | United Therap | 2009 |
| Glycopyrrolate + Formoterol fumarate | pMDI | Bevespi Aerosphere | AstraZeneca | 2016 | |
| Budesonide, glycopyrronium, and formoterol (PT010)* | pMDI | NA | Pearl Therapeutics | Clinical Trial Phase III |
A major problem with current inhalers is the significant deposition of drug particles in the oropharyngeal region and upper airways (Ibrahim, Verma and Garcia-Contreras, 2015). In addition, lack of coordination between device actuation and patient inhalation (hand-breath coordination) increases the deposition in the upper airways leading to insufficient amounts of drug being delivered to the deep lungs (Scichilone, 2015). Fink and Rubin have estimated that improper inhaler maneuvers have a huge economic impact, with roughly $7–15.7 billion wasted, with no benefit to the health-care system or the patient population (Fink and Rubin, 2005).
pMDIsFor pMDIs, to overcome the hand-breath coordination problem, the use of add-on devices such as a spacer or valve-holding chamber is recommended, especially for children and the elderly (Kunda et al., 2017). These add-on devices increase the time between device actuation and inhalation allowing patients to inhale drug over longer periods of time thereby enhancing drug delivery to the deep lungs and decreasing deposition in the oropharyngeal regions and upper airways (Zhou et al., 2014; Kunda et al., 2017). More recently, pMDIs have been designed to operate on breath-activation rather than hand-breath coordination (Newman, 2005; Zhou et al., 2014).
NebulizersNebulizers are recommended for patients who are unable to perform proper hand-breath coordination or perform a forceful inspiration; further, these devices work well for diseases which require higher inhalation doses (eg. CF patients). Three main types of nebulizers are available on the market and are differentiated by their aerosolization mechanism: Jet nebulizers, ultrasonic nebulizers, and vibrating mesh nebulizers. The vibrating mesh nebulizer overcomes some of the disadvantages observed with the first two nebulizer types, like lowering the total treatment time, minimal residual drug volumes, and better efficiency in drug delivery to the lung. However, the complicated operational procedures for nebulizers along with their high cost remains a barrier to better patience compliance (Ari and Fink, 2013; Chan et al., 2014; Ibrahim, Verma and Garcia-Contreras, 2015; Tashkin, 2016).
DPIsFor these devices, the properties of the dry powder formulation, the device design, and the patient inspiratory flow rate determine the efficiency of the inhalation product and the drug deposition to the deep lungs (Islam and Cleary, 2012). In addition, the inspiratory flow rate must be maintained for the duration of inhalation, with deep and forceful breaths, to de-aggregate drug/lactose carrier particles and facilitate drug deposition to the deep lung (Scichilone, 2015). Moreover, a ‘deep and forceful’ inspiration may be difficult to achieve for patients suffering from asthma or COPD, thereby limiting the use of passive DPIs. Passive DPIs require the patient’s inspiratory effort to first disperse the powder in the device and subsequently deliver the formulation to the deep lung. In addition, a rapid inspiration is likely to cause higher oropharyngeal deposition of the drug. Recent progress in the development of active DPIs that use an external energy source, such as compressed air or electrical vibration, may help in powder dispersion and de-agglomeration and thus increase drug deposition to the lungs. However, the major obstacle to the success of active DPIs is their high cost and portability (bulky devices) (Zhou et al., 2014). In addition, specific attention must be given to the pediatric and geriatric population since most DPI devices are designed for the adult population.
2.5 Progress in DPIs: The story of insulinIn recent years, the increasing worldwide diabetic population has motivated many pharmaceutical companies to focus on developing an inhalable insulin product that is fast-acting and can be administered without needles. In 2006, the FDA approved the first inhaled insulin, Exubera®, which was developed and marketed by Nektar Therapeutics and Pfizer laboratories, respectively (Mack, 2007). It is thought that Exubera® was withdrawn in 2007 because of poor patient compliance due to the cost and bulkiness of the device. Perhaps this may be explained by the reported lack of input from the physicians and patients on the design of the device before marketing Exubera®; most of the patients felt uncomfortable using the bulky device, which was almost the size of a flashlight (Mack, 2007).
With lessons learned from the failure of Exubera®, MannKind Corp. developed a smaller inhaled insulin product, Afrezza®, that could easily fit the palm of the patient (later marketed by Sanofi). Afrezza® was approved by the FDA in early 2015 for patients to help control their blood sugar levels during mealtime. The product reaches peak insulin levels in 12–15 min and remains active for up to 3 hours (Hoskins, 2017). However, Sanofi recently withdrew from marketing Afrezza® due to mediocre profits. Dance Pharmaceuticals is currently testing an inhalation insulin product, Dance-501, that delivers a gentle mist of recombinant human insulin using a vibrating mesh micropump technology (developed by Aerogen, Inc.). The story of insulin reflects the challenges associated with successful development of a DPI inhalation product which depends on the formulation, device, delivery, packaging, and proper marketing toward the physicians and patients. Despite the uncertainty observed with the development of an inhaled insulin product, there is renewed interest in exploring dry powder delivery of macromolecules for local and systemic delivery via inhalation.
Animal models are crucial to evaluate the fate of inhaled material and providing valuable information for inhalation product development. When selecting the appropriate animal model, many parameters are considered, including cost, disease pathology, and immunological similarity to humans. Although the data obtained from preclinical studies is valuable in advancing inhalation drug delivery, extrapolation to humans is not straightforward due to the differences in the nasal, tracheobronchial, and the deep lung region across species (Phalen et al., 2008; Fernandes and Vanbever, 2009). For this reason, the similarity of the structure of the respiratory tract of the animal model to humans should be a significant parameter in choosing an appropriate animal model for preclinical studies (Phalen et al., 2008).
Human breathing differs from that of rodents. The latter, such as mice and rats, are obligatory nose breathers and are unable to perform mouth breathing, while adult humans can breathe through the nose and the mouth (Fig. 2). In contrast to adult humans, newly born human infants are obligate nose breathers during the first 2–6 months of life (Fig. 2) (Rubin and Williams, 2014; Amirav et al., 2015; McGregor, 2017). The inability to inhale via the mouth limits the relevance of lung deposition data obtained through preclinical animal models that utilize whole-body and nose-only exposures. When using a nose-only or whole-body exposure systems, particles larger than a few microns are trapped in the nasal-pharyngeal region of the rodents and do not necessarily represent the true lung deposition profile observed in humans (Phalen et al., 2008). Another difference between humans and rodents is the presence or absence of respiratory bronchioles. The absence of respiratory bronchioles in the commonly used preclinical models results in different region-specific lung deposition data compared to humans. The clearance mechanism of inhaled insoluble particles also varies in the presence or absence of respiratory bronchioles (Phalen and Mendez, 2009).

Upper airway structural differences between human infants, adults, and rodents.
In addition to the differences in the respiratory physiology and anatomy, cells present in the lung may also vary between humans and the animal model used. It is estimated that human alveolar macrophages are greater in number and are two-to-three fold larger in size than rodent alveolar macrophages (Stone et al., 1992). Additionally, humans have intravascular macrophages, cells that attack pathogens entering the lungs through the bloodstream, that are absent in rodents (Balhara and Gounni, 2012). These notable differences between humans and the most commonly employed rodent-based animal models call for a more cautious approach in extrapolating pulmonary drug delivery and disposition results from preclinical studies to humans.
3.2 Preclinical inhalation methodologiesOne of the greatest obstacles to the successful transition of dry powder products from the research bench to the clinic is the lack of preclinical pulmonary delivery devices. Ideally, preclinical devices should work on similar mechanisms to that of human pulmonary delivery devices. Currently, inhaled medicines are delivered with three types of devices in humans: pMDIs, DPIs, and nebulizers, as discussed in a previous section (Hickey, 2013). However, human inhaler devices work on principles that differ from those used in preclinical models; this usually leads to variable deposition profiles of the agent in the respiratory tract.
The pulmonary delivery devices currently available for preclinical evaluation of aerosols can be categorized into passive and direct inhalation devices. Passive inhalation describes the ability of the animal to breathe an aerosol normally without anesthesia, whereas direct inhalation devices force the aerosol into the upper respiratory tract of the animal, usually under anesthesia. While both types of devices are widely used in the aerosol preclinical research field, the choice of device type, passive or direct, may be appropriate to certain experimental designs.
Passive inhalation delivery devices include whole-body inhalation chambers and head- and nose-only chambers (Fig. 3A, B). These devices do not require surgery or that the animal be anesthetized during the aerosol delivery. Further, passive aerosol delivery devices are considered more physiologically-relevant because the animal breathes in the aerosol normally with tidal breathing, and without any external force delivering the aerosol into the lungs. For small animals such as rabbits and rodents, the inhaled aerosol passes through the nasal cavity before reaching the lungs, as these animals are obligate nose breathers (Fig. 2) (Hoppentocht et al., 2014b). The passage of the aerosol via the nasal cavity will affect the amount, as well as the site, of aerosol deposition in the lung. Importantly, this deposition will be different compared to humans who inhale via the oral cavity using the existing oral inhalation devices. The fraction of dose deposited in the lung is further altered by the complex nasal turbinate anatomy of rodents, compared to a simple structure observed in humans (Table 2).
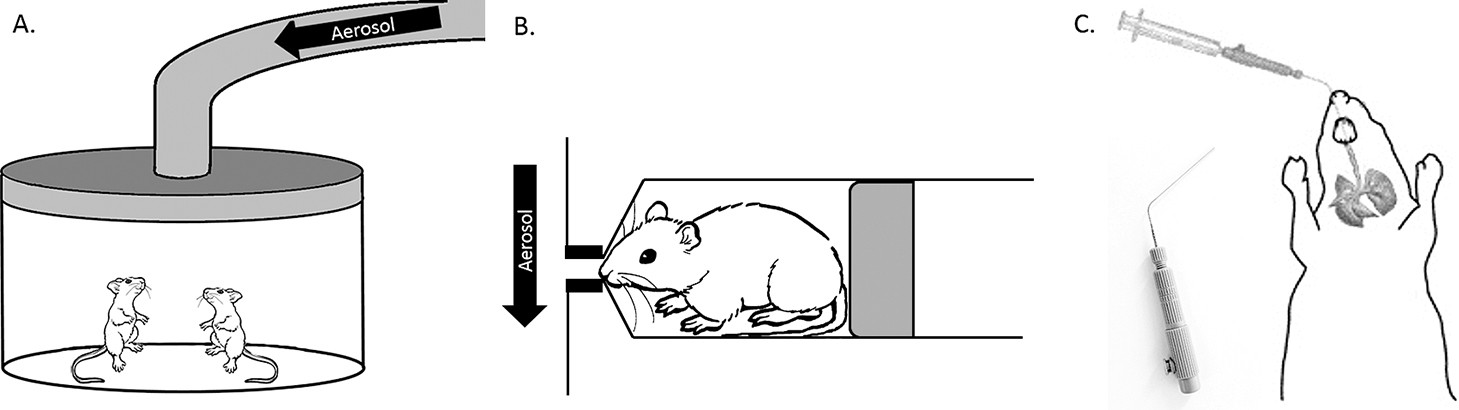
Preclinical dry powder delivery devices. Passive inhalation devices include A) Whole body and B) Nose- and head-only devices. Direct inhalation methods require intubation of the rodent trachea. Shown in C) An intubated rodent with the Penn Century dry powder InsufflatorTM (inset: InsufflatorTM).
| Characteristics | Human | Rhesus monkey | Beagle dog | Rabbit | Guinea-pig | Rat | Mouse | Ref. |
|---|---|---|---|---|---|---|---|---|
| Body Weight (kg) | 70 | 38 | 10–15 | 2.5–3.5 | 0.4 | 0.25–0.35 | 0.02–0.04 | (Fernandes and Vanbever, 2009) |
| Nose and/or mouse breather | Nose and mouth breather | Nose and mouth breather | Nose and mouth breather | Obligate nose breather | Obligate nose breather | Obligate nose breather | Obligate nose breather | (Fernandes and Vanbever, 2009) |
| Nasal Anatomy | ||||||||
| Turbinate complexity | Simple | Simple scroll | Very complex | Complex scroll | Complex scroll | Complex scroll | Complex scroll | (Schreider and Raabe, 1981; Fernandes and Vanbever, 2009) |
| Pulmonary Anatomy | ||||||||
| Lung weight (g) | 1000 | — | 100 | 18 | 3.2 | 1.5 | 0.12 | (Brewer and Cruise, 1997; Fernandes and Vanbever, 2009) |
| Lung symmetry | dichotomous | dichotomous | monopodial | monopodial | monopodial | monopodial | monopodial | (Fernandes and Vanbever, 2009) |
| Trachea length/diameter (cm) | 12/2 | 3/0.3 | 17/1.6 | 6/0.5 | 5.7/0.4 | 2.3/0.26 | 0.7/0.12 | (Hoyt, Robert F. et al., 2007; Fernandes and Vanbever, 2009; Purser, 2010) |
| Lung volume (mL) | 4341 | 204 | 736 | 79.2 | 13 | 8.6 | 0.74 | (Fernandes and Vanbever, 2009; Asgharian et al., 2012) |
| Number of Alveoli (×106) | 950 | 81.8 | 1040 | 135 | 69 | 43 | 18 | (Fernandes and Vanbever, 2009; Asgharian et al., 2012) |
| Diameter of Alveoli (μm) | 219 | — | 126 | 88 | 65 | 70 | 47 | (Fernandes and Vanbever, 2009) |
| Alveolar macrophages (×106) | 5990 | — | 3940 | 142 | 58.8 | 29.1 | 2.9 | (Fernandes and Vanbever, 2009) |
| Respiratory parameters | ||||||||
| Respiratory rate (min−1) | 12 | 38 | 23 | 51 | 90 | 85 | 163 | (Fernandes and Vanbever, 2009) |
| Tidal volume (L/min) | 400–616 | 20–21.2 | 11.4–16.6 | 15.8 | 1.72–1.75 | 0.87–2.08 | 0.15–0.18 | (Fernandes and Vanbever, 2009) |
| Mucus clearance rate (mm/min) | 3.6–21.5 | 7.5.21.6 | 3.2 | 2.7 | 1.9–5.9 | — | (Fernandes and Vanbever, 2009) | |
| Particle size for deep lung delivery (μm) | 1–5 | 1–3 | 1–3 | — | — | 3.5 | 3 | (Fernandes and Vanbever, 2009; Asgharian et al., 2012) |
Modified from Fernandes et al. (Fernandes and Vanbever, 2009)
Whole-body chambers expose the whole animal to an aerosol atmosphere (Fig. 3A). These chambers have the advantage of housing several animals at once and the chambers can include food and water access. Commercially available devices include the Glas-Col® inhalation exposure systems and other units from TSE-systems and Shibata Biotechnology (Yi et al., 2013; Chung et al., 2015). Other advantages of whole-body exposure chambers include the ability to house animals for long periods if the experimental design requires long-term exposure, and repeated dosing is also possible. However, drawbacks of the whole-body exposure system include extra-pulmonary exposure through other administrations routes such as skin and oral/GI tract, the requirement of large amounts of drug or dosing material, and difficulty quantifying and characterizing the dose delivered into the lung per animal (Table 3) (Cryan, Sivadas and Garcia-Contreras, 2007; Wong, 2007; Fernandes and Vanbever, 2009; Nahar et al., 2013). Ultimately, these chambers make excellent devices to study environmental exposures that require long-term dosing.
| Methodology | Tissue Distribution | Tools required/formulation | Benefits | Drawbacks | Ref. |
|---|---|---|---|---|---|
| Passive methodologies | |||||
| Whole body | Respiratory tract, GI tract, skin/fur, nasopharynx | Aerosol generator and exposure chamber, | Physiologically relevant, animals can be housed in chambers, long-term exposure capable, accommodates large group sizes | Other exposure routes, large amounts of material required, homogeneous air flow required, difficult to characterize deep lung dosing | (Cryan, Sivadas and Garcia-Contreras, 2007; Wong, 2007; Fernandes and Vanbever, 2009; Nahar et al., 2013) |
| Head Nose-only | Respiratory tract, GI tract, nasopharynx | Aerosol generator with compatible restraining tubes | Reduced extra-pulmonary exposure, reduced material requirements, suitable for repeated dosing | Animals are restrained, possible heat buildup, probable stress on animal and potential suffocation, labor intensive, difficult to characterize deep lung dosing | (Cryan, Sivadas and Garcia-Contreras, 2007; Wong, 2007; Fernandes and Vanbever, 2009; Nahar et al., 2013) |
| Direct methodologies | |||||
| Intratracheal Instillation | Trachea, Respiratory tract | Penn Century InsufflatorTM or Braintree Scientific Lung intubation system, plastic intubation needle | Direct dosing, Efficient dosing | Anesthesia, tracheal damage, technically difficult, time-intensive | (Cryan, Sivadas and Garcia-Contreras, 2007; Wong, 2007; Morello et al., 2009; Duret et al., 2012; Hoppentocht et al., 2014b) |
| Tracheostomy | Trachea, Respiratory tract (diffusion) | Surgery tools | Direct dosing, Efficient dosing | Anesthesia, surgery, not suitable for repeated or long-term dosing, labor intensive | (Lakatos et al., 2006; Fernandes and Vanbever, 2009) |
Modified from Wong et al. (Wong, 2007)
Head- and nose-only exposure chambers are like the whole-body chamber in that animals are not anesthetized during exposure and are able to breathe in a more physiologically- relevant environment. However, in contrast to the whole-body chamber, animals are held immobilized in an exposure tube with the animal’s nose or head exposed to an airflow containing the dosing agent (Fig. 3B). Such an exposure chamber decreases extra-pulmonary exposure by other delivery routes and decreases the total amount of material needed for the experiment. However, several independent studies have shown that 80–90 % of inhaled aerosols are deposited into the nasopharynx and upper respiratory tract, despite the aerosols being in the respirable range (Society of Toxicology report, 1992; Kaur et al., 2008; Kuehl et al., 2012; Hoppentocht et al., 2014b). Very little of the aerosol reaches the deep lung, and therefore quantification of dose can be difficult. Other flaws of the system include stress on the animal, due to restraints and lack of access to food and water, as well as suffocation (rare occasion) if the animal moves around in the tube or the chamber generates heat (Table 3).
Direct inhalation devices include intratracheal methods of delivery and tracheostomy. These methods of aerosol delivery are considered less physiologically-relevant as the animal is anesthetized while the aerosol is forced directly into the trachea, rather than through normal inspiration. However, unlike passive inhalation methodologies, the quantification of the dose delivered into the lung is much easier with these devices because the nasopharynx is bypassed and the aerosol is forced directly into the trachea and upper lung airways (Hoppentocht et al., 2014b).
Intratracheal instillation (also called intratracheal/endotracheal intubation or aerosolization) is the most utilized method of direct administration of therapeutics into the respiratory system of rodents. Several tools have been developed for this type of delivery; however, options become limited when delivering dry powders compared to liquid aerosols. Dry powder tools include the Penn Century InsufflatorTM (Fig. 3C) and the BioLite Intubation System from Braintree Scientific, Inc (Walters et al., 2004; Morello et al., 2009). Using these devices an anesthetized rodent is intubated with an insufflator tube or catheter as far as the first bifurcation of the lungs (Fig. 3C). The dry powder is then aerosolized directly into the lung using a syringe or pipette bulb. The methodology is technically difficult and time intensive in smaller animals (i.e. mice and rats), however, repeated dosing and long-term studies are possible (Table 3).
Major drawbacks of the intratracheal instillation technique include the requirement of anesthetization of the animal and a lack of universal availability of devices in the market. Repeated use of anesthesia can alter the physiology of animals (Balcombe et al., 2004; Hildebrandt et al., 2008), and makes intratracheal instillation a challenging method for long-term dosing studies. The lack of a universally accepted dry powder delivery device among different research laboratories leads to in-house fabrication of devices which result in variable delivery efficiency in published animal models. The Penn Century InsufflatorTM was the most-published direct inhalation (for dry powders), until recently when the company closed. Now this device has become difficult to purchase and researchers are developing alternatives (Durham et al., 2017).
The insufflator was not without its problems however, and had been evaluated in several animal models using variable dosing parameters. Penn Century stated that the insufflator was designed to hold 1–4 mg of dry powder. However, Morello et al. showed that powder doses above 350 μg delivered with the InsufflatorTM could cause immediate death in mice (Morello et al., 2009). Furthermore, the same study showed that use of more than 250 μL of air (4–5x) resulted in mouse death. In contradiction to these studies, Duret et al. published an evaluation of the insufflator with powder doses ranging from 0.5 mg to 4.3 mg and suggested that dose recovery (drug amount that reached the lung lobe) was proportional to the amount insufflated into the lung (Duret et al., 2012). Hoppentocht and colleagues showed that powder aerosolized with less than 500–1000 μL of air led to significant powder remaining in the insufflator and had decreased lung deposition efficacy (Hoppentocht et al., 2014b). In rats, intubation with the insufflator alone (no drug delivered into the lung) was shown to cause lung trauma (Guillon et al., 2012).
Tracheal impaction and deposition is another problem associated with powder delivery using direct intubation strategies. The insufflator intubates the trachea up to the first bifurcation of the lungs. The powder is aerosolized through the firm depression of a syringe plunger attached to the insufflator (Fig. 3C). The force required to deagglomerate and aerosolize the powder from the insufflator results in an excessive force exerted on the particles, such that their momentum carries them directly into the walls of the trachea due to inertial impaction. Duret et al. and Tonnis et al. both showed independently that only about 65 % of the powder dose emitted from the insufflator reaches the lungs (Duret et al., 2012; Tonnis et al., 2014). Particles deposited onto the tracheal walls are rapidly cleared through mucociliary clearance and swallowed by the animal. In addition to the dose lost through tracheal impaction, a certain amount of powder is lost through exhalation due to the return airflow caused by overpressure within the lungs due to the sudden burst of air blown via the insufflator (Tonnis et al., 2014). Price et al. recently showed that poor lung deposition might also be associated with the humid lung environment in the rodent lung leading to powder agglomeration and retention in the insufflator (Price et al., 2017).
The only other methodology reported for direct delivery of dry powders is tracheostomy (Lombry et al., 2004; Wong, 2007; Fernandes and Vanbever, 2009). This method involves a surgical incision to expose the trachea and the placement of powders into a cannula that is inserted towards the lung or directly into the upper respiratory tract. The advantages of this method include efficient use of dosing material and easy characterization of deep lung dosing (Wong, 2007). However, this method has the drawback of invasiveness and cannot be used for long-term or repeated studies (Table 3) (Wong, 2007; Fernandes and Vanbever, 2009).
3.3 Dry powder formulation for preclinical pulmonary deliveryDry powder flow properties and lung distribution patterns vary depending on the device with which they are delivered, the animal model used, and the formulation characteristics of the powder itself (Fig. 4) (Foster et al., 2001; Fernandes and Vanbever, 2009). Foster et al. showed that the mucociliary clearance of aerosolized insoluble colloidal particles and its distribution in a mouse lung were dependent on the delivery device (Foster et al., 2001). Nose-only administration delivered significantly fewer particles to the lung than intratracheal instillation and the deposited particles were cleared much more efficiently from the lung within 6- and 24-hour observational timepoints than particles delivered by intratracheal delivery. Further, small operational changes within a delivery technique using a particular delivery device can change the lung distribution patterns within the animal (Foster et al., 2001; Wong, 2007; Hasegawa-Baba et al., 2014). Hasegawa-Baba and colleagues showed a significant difference in pulmonary distribution patterns generated by intratracheal instillation depending on the angle at which the animal was intubated, the speed at which the drug was aerosolized into the lungs, and the volume of the dose delivered (Hasegawa-Baba et al., 2014). These differences in the operation of preclinical devices are inevitable based on individual operators working in different laboratories.
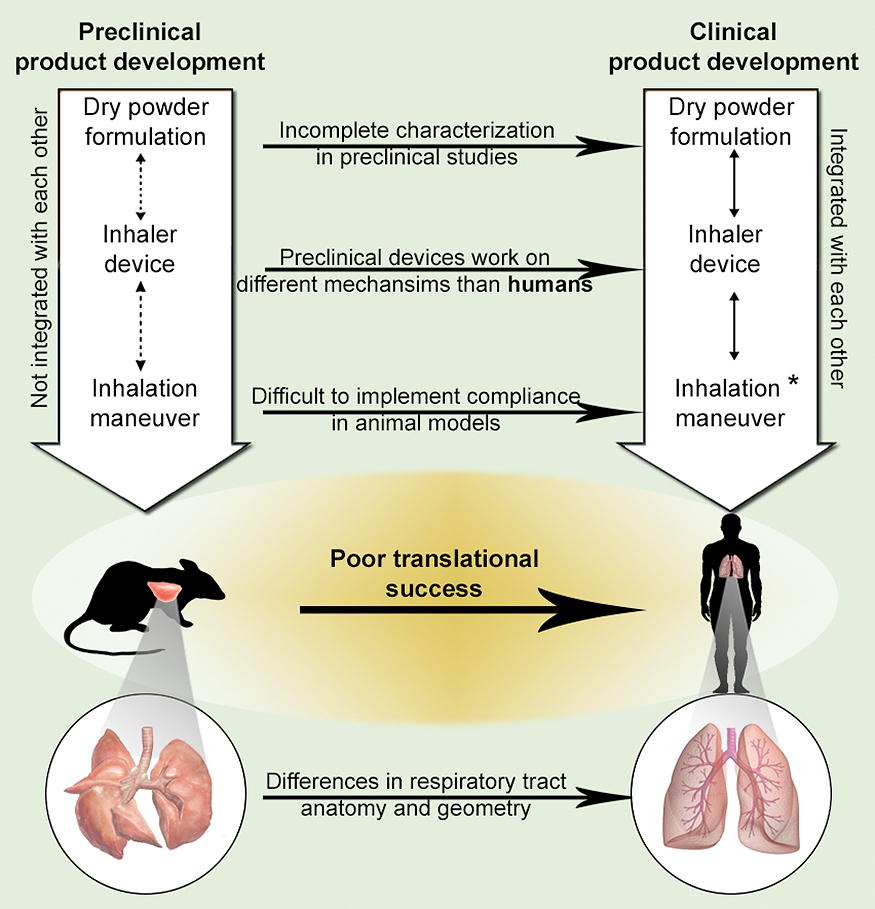
The reasons for poor translational success of DPI products from preclinical studies to human. *Integration of inhalation maneuver remains a problem in infant and pediatric human populations.
Historically, small and large animal models have been used for inhalation studies, and as mentioned earlier, these models differ greatly in their respiratory physiologies. The textbook definition of the optimal size range of particles delivered to the deep lung is 1–5 μm, without distinction between the animal models used. However, it is likely that the optimal pulmonary size range changes with the animal, the device used, and even by the device operator as mentioned above. Kuehl and colleagues delivered polydisperse powders (0.5 to 5 μm) to rats and mice using a nose-only inhalation system and observed different powder deposition patterns in the lung, as a function of particle size, within these two animal models (Kuehl et al., 2012). Phagocytosis and immune cell recruitment in response to pulmonary vaccines and therapeutics may also differ between animal models based on the macrophage numbers in the lung (Fernandes and Vanbever, 2009). Likewise, differing mucociliary clearance rates between animal species may affect drug retention in the lung and therefore efficacy, especially in long-term treatment studies (Fernandes and Vanbever, 2009).
Dry powders for pulmonary delivery may require different characterization for preclinical studies, separate from what goes into clinical trials. The lung distribution of the powder delivered by the insufflatorTM varies based on the powder properties and how it was formulated (Duret et al., 2012; Hoppentocht et al., 2014b). Powders with increased hygroscopicity may be difficult to deliver by intratracheal instillation, as the humid environment of the trachea may cause powder aggregation and clogging of the intubation tube (Price et al., 2017). Lastly, because dosing varies greatly between different methods of pulmonary delivery, i.e. passive and direct, it is critical to optimize the amount of powder delivered based on the device and the animal model used.
Significant progress has been made in the dry powder field (both in the formulation and device development) for humans since DPIs were first introduced almost half-century ago (Table 1). In this section, we will discuss some of the recent innovations in powder formulation, and inhaler devices that could improve not only drug deposition in the lung but patient adherence to treatment as well. We will end by discussing how these innovations can be applied toward animal inhalation studies.
4.1 Formulation innovationsDry powders for pulmonary delivery were historically made using the ‘top-down’ approach of milling larger crystalline particles into a size appropriate for inhalation. This led to irregularly shaped particles that were cohesive and promoted particle aggregation. Although adding inert carrier particles such as lactose or mannitol to drug particles decreased particle cohesiveness to a variable extent, it also reduced delivery efficiency to the lung due to an increase in the total inhaled powder volume (Rubin and Williams, 2014). Newer formulation technologies have focused on engineering spherical particles in the respirable range where the active ingredient is encapsulated within an inert matrix, usually using a ‘bottom-up’ approach (precipitation and solvent evaporation techniques). Such an approach allows for greater control of particle size and polydispersity, morphology, porosity, density, and surface energy (Weers, Clark and Challoner, 2004). Lower contact points for spherical particles has helped in particle dispersion without the need for carrier particles; this has improved the delivery efficiency of the API by significantly decreasing the total volume of dry powder inhaled by the patient. Carrier-free spherical particles have been manufactured using techniques such as supercritical fluid, spray-freeze drying, and spray drying. These techniques can formulate liposomes and polymeric micro- and nanoparticles in the appropriate size range for pulmonary delivery. Liposomal formulations are usually well-tolerated and non-immunogenic when delivered into the lung since they can be prepared using surfactant phospholipids that are endogenous to the lung, for eg. dipalmitoylphosphatidylcholine (DPPC) (Cipolla et al., 2014).
Spray drying produces large porous particles in the respirable range with reduced particle aggregation during aerosolization (Edwards et al., 1997). Further, porous particles have achieved significantly higher drug loading; PulmoSpheresTM, manufactured by an emulsion-based spray drying process, has achieved more than 90 % tobramycin loading by mass (Geller et al., 2011). Pulmo-SpheresTM offer substantially improved lung deposition efficiency and more convenient administration over the nebulized delivery of tobramycin. Spray freeze-drying is used to produce dry powders for pulmonary delivery that have lower aggregation capability. However, due to their high porosity, spray-freeze-dried particles allow for low powder volumes to be loaded in DPIs (Saluja et al., 2010). Supercritical fluid technology is another bottom-up technology that generates uniformly sized particles for pulmonary delivery (Sacchetti and Van Oort, 2007). For sreviews on dry powder formulation for pulmonary delivery, the readers are directed to articles that broadly cover these topics (Yang, Chan and Chan, 2014; Carvalho et al., 2015; Hickey, 2018).
4.2 Device innovationHuman DPI devices are categorized into passive and active inhalers as discussed earlier. Passive inhalers require the patient’s inspiration to deaggregate powders into the respirable range. Powders loaded in passive inhalers should, therefore, exhibit improved flow and dispersion properties based on the patient’s inspiratory strength. Conversely, active inhalers provide the energy for powder dispersion and are not dependent on the patient’s peak inspiratory flow rate. Newer passive inhaler devices enhance powder de-agglomeration and are less dependent on the patient’s inspiration. Such technologies include reverse flow cyclone technology (Harrison et al., 2011), mesh-sieving technology (Friebel et al., 2010), flutter-induced powder dispersion (Selvam et al., 2010). Although these device designs have minimized dependency on the patient inspiration to disperse powders in the inhaler device, low flow rates could still be a concern in patients with limited lung function or a reduced tidal volume, including children, the elderly, and patients suffering from cystic fibrosis. Current DPIs use carrier-based dry powders that worsen the above concerns, in addition to the significant deposition in the mouth and throat area observed with such powders, irrespective of the inhaler used. Carrier-free dry powders were evaluated using the HandiHaler® device and turbulence was shown to be their primary deaggregation mechanism (Longest et al., 2013). Highly effective passive inhalers may be required for carrier-free powders in order to improve the respirable fraction (10–70 %) currently achieved with the marketed DPIs (Islam and Cleary, 2012; Behara et al., 2014). Active inhaler devices disperse powders based on an external energy source such as compressed air, electrical vibration, and mechanical barriers. Although a few active dry powder inhalers have been clinically tested (Hoppentocht et al., 2014a), their widespread use in the future may be hindered due to their complexity, expense, failure due to the requirement for an external energy source, and most importantly, poor patient compliance due to the numerous operational steps required for inhalation (Smutney, Grant and Kinsey, 2013; Chan et al., 2014; de Boer et al., 2017).
Drug delivery efficiency to the lung has shown significant improvement when a powder inhaler is evaluated in combination with a specific formulation technology in human trials before being marketed (Frijlink and De Boer, 2004; Smutney, Grant and Kinsey, 2013). The TOBI PodhalerTM, when tailored with tobramycin PulmoSpheresTM, delivered up to 60 % of the total dose into the lung of healthy human subjects (Duddu et al., 2002); a significant increase from the usual 1–2 % delivery efficiency observed with earlier DPIs. Thus, the ability to characterize the inhaler device along with the formulation is critical for improving the amount of powder delivered into the lung (Fig. 4 & 5).
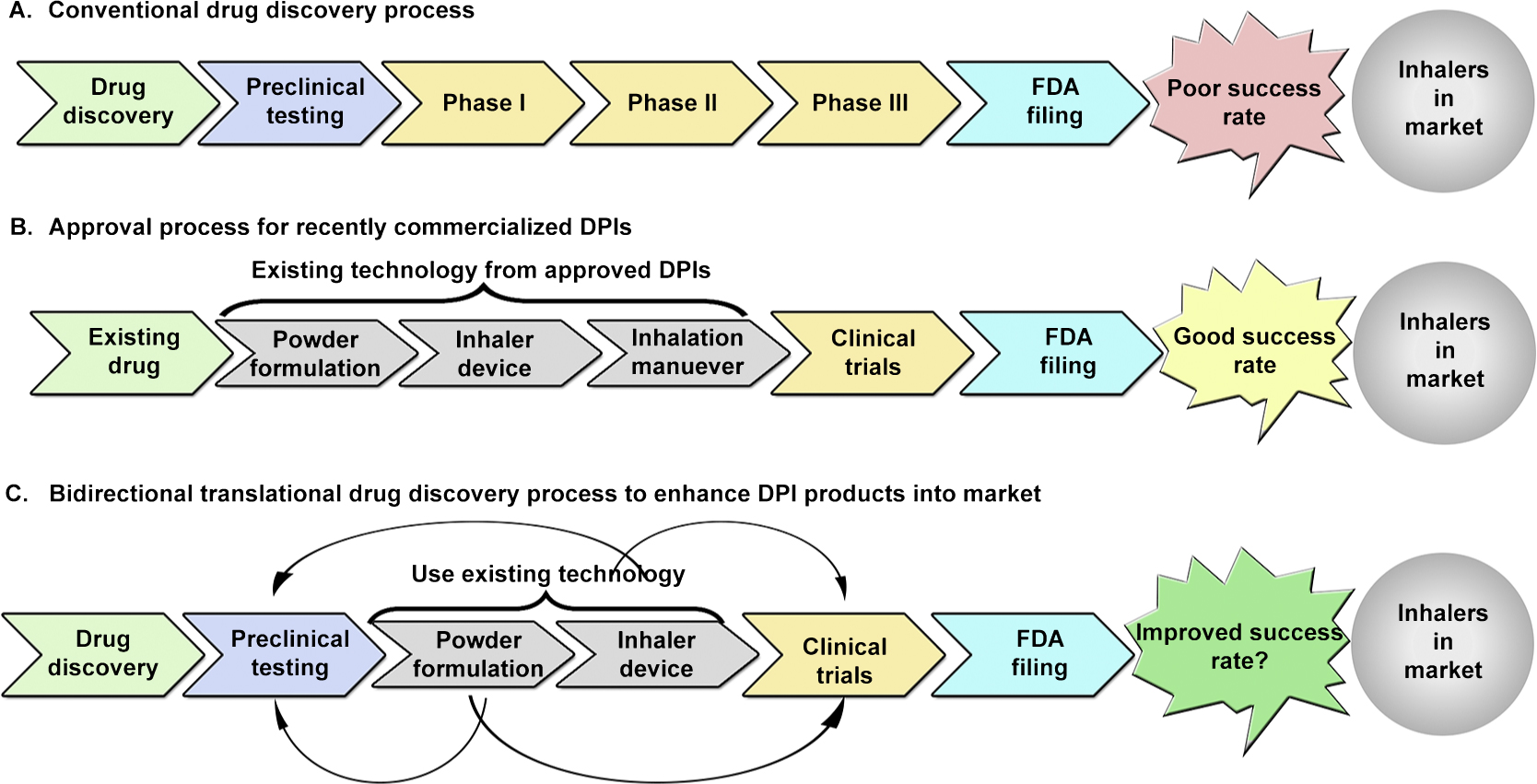
Linear (conventional) drug discovery versus bidirectional drug discovery.
The ability to manipulate the patient’s ‘inhalation maneuver’ while using a DPI has shown to improve the drug delivery efficiency to the lung. Conversely, incorrect inhaler use is a major factor in poor disease outcomes; up to 68 % of patients using DPIs do not use the device correctly (Fink and Rubin, 2005). Inhalation maneuver includes controlling the inhalation flow rate, holding breath for 10s or more, and varying the inhalation volume and inspiration time (Haidl et al., 2016). The patient’s ability to hold their breath has shown to improve lung deposition efficiency by allowing the particles to settle in the deep lung region by diffusion (Horváth et al., 2017) and possibly leading to minimal exhalation of particles. Inhalation maneuver becomes even more critical for DPIs, compared to pMDIs and nebulizers, since the ability to vary the inspiration volume and time will dictate the amount of powder dispersed and ejected from an inhaler. However, manipulating the inhalation maneuver in human infants and rodents is challenging; both these groups are obligate nose-breathers compared to human adults (Fig. 2) and this results in significant powder deposition in the nasopharyngeal region rather than in the lower respiratory tract (DiBlasi, 2015).
4.3 Implementation of lessons learned from humans to animalsMany preclinical studies using dry powder pulmonary delivery for drugs, vaccines, and immunotherapies have been conducted in the last two decades. However, the translational success from these preclinical studies to human trials has been abysmal (Fig. 5). The poor success in clinical translation may be associated with the complexity of the pulmonary route of administration, especially when compared to the parenteral route of administration. For parenterally delivered products, similar formulation and devices are used across animals and humans. For example, a drug solution administered using a syringe and needle in preclinical studies will not require significant modifications to the formulation (solution) or device (syringes and needles) when subsequently evaluated in humans. However, for pulmonary delivery, differences in lung anatomy, respiratory physiology, formulation characteristics, and device type between preclinical and clinical studies could have significant implications for translation success (Fig. 4). Due to these differences, drug deposition patterns in the lung and drug clearance rates can vary between humans and animals (Cryan, Sivadas and Garcia-Contreras, 2007). In addition, variable drug losses are observed in the delivery device and in the host (both humans and animals). The complex nasal passage in rodents usually leads to significant powder deposition in the nasal passage, compared to the deposition observed in the oral passage in humans, when delivering dry powders to the lung. Furthermore, the compatibility of a powder formulation in combination with a specific inhalation device is not characterized in preclinical studies. This is due to the scarcity of inhalation devices available for preclinical studies (see preclinical inhalation methodologies section); in addition, as discussed previously, preclinical devices work on different mechanisms than that of human inhalers and do not take into account the lung anatomy and physiology of the preclinical model (see animal models section).
In the drug discovery process, preclinical studies precede human studies. However, recent drugs approved for DPI use were previously marketed as pMDIs and nebulizers, or utilized existing dry powder formulation technology and inhaler devices (Fig. 5B); very few of these DPIs went through the linear (conventional) drug discovery process involving preclinical studies (Fig. 5A). This shows that the translational success for pulmonary drug development can be significantly improved by including reverse- and forward-signaling loops (bidirectional) in the linear drug discovery process based on a feedback mechanism (Fig. 5C) (Wehling M., 2009). Although using such a feedback system cannot replace any of the steps required in the drug discovery cycle, it could increase the likelihood of a DPI product reaching the market.
The important and positive lessons learned from dry powder formulation and characterization, inhaler design, and subsequent evaluation in humans should be employed in preclinical studies to the extent possible (Lightfoot et at., 2017). In addition, a new drug can use the existing technology with regards to powder formulation and inhaler device in a forward-signaling loop in the discovery process (Fig. 5C). Such a feedback system will allow better collaboration between the basic scientist, industry partners, and clinicians working on preclinical inhalation studies, formulation and device development, and evaluating novel DPIs in humans, respectively. If possible, we need to develop preclinical inhaler devices that more closely mimic the devices being tested in humans. However, we need to also acknowledge the limitations of animal models with regards to their translational potential. This could be particularly true for drugs and biologics for pulmonary delivery.
Dry powder inhaled therapeutics have solved many problems associated with liquid therapeutics and their associated inhalers. However, drug development for dry powder formulations and inhalers have consistently faltered as they transition to the clinic, and de novo dry powder drug development is sparse when a linear drug discovery process is followed. The low success rate of these products may be due to the lack of innovation in other areas of preclinical pulmonary drug development. Current preclinical pulmonary delivery methodologies do not model human inspiration or current DPI mechanisms. Further, dry powder formulations are never modulated to fit preclinical models and devices; they are formulated with only human drug delivery in mind. Thus, innovation in dry powder therapeutics has become unidirectional in terms of development and very few products make it to market. To increase the success of dry powders in the pharmaceutical industry, efforts must be shifted to fix the current problems with preclinical development, and innovation must become bi-directional. This will create a system where novel research and discovery can inform preclinical and clinical drug development.
Dominique N. Price
Dominique Price is a medical student at the University of New Mexico Health Science Center, USA. She received her Ph.D. in Biomedical Sciences in 2016 from the University of New Mexico, USA. She has a broad research background in pulmonary delivery and infectious disease, especially diseases of the lung. She is continues to be an active researcher in laboratory of Dr. Pavan Muttil in the College of Pharmacy, University of New Mexico, USA. Her publications and research interests include a wide variety of research topics including localized delivery of pulmonary therapeutics, stabilization of bacterial vaccines and biologics, and interactions of environmental bacterial with immunization efficacy in tuberculosis.
Nitesh K. Kunda
Nitesh Kunda is a Post-doctoral fellow in the Department of Pharmaceutical Sciences at the College of Pharmacy, University of New Mexico, USA. He received his Ph.D. in Formulation and Drug Delivery in 2014 from Liverpool John Moores University, UK. He has extensive research experience in drug formulation, as well as drug and vaccine delivery, specifically pulmonary delivery. His research interests lie in nanotechnology in drug delivery, design and development of vaccine delivery systems, proteins and gene formulations, microneedle formulations, synthesizing polymeric nanoparticles and attaching targeting moieties for site specific delivery. His publications include a wide variety of research topics including formulation and delivery of drugs and vaccines, lung delivery, and stabilization of bacterial-based and viral-based vaccines. He is an active member of professional organizations such as the Royal Society of Chemistry, the Aerosol Society, American Association of Pharmaceutical Scientists, and the British Society of Nanomedicine amongst others.
Pavan Muttil
Dr. Muttil is an Associate Professor in the Department of Pharmaceutical Sciences at the University of New Mexico, NM, USA. He obtained his Ph.D. in aerosol drug delivery from Central Drug Research Institute, Lucknow, India. Dr. Muttil did his post-doctoral research in aerosol drug and vaccine delivery at the Eshelman School of Pharmacy, University of North Carolina, Chapel Hill. He was the recipient of a Bill and Melinda Gates Grand Challenges Exploration grant in 2012. Research in the Muttil Laboratory is focused on developing drug and vaccine formulations for inhaled, oral, and transdermal delivery systems for infectious diseases and cancers. These novel formulations are evaluated in animal models for their efficacy as a needle-free delivery strategy.