2019 年 36 巻 p. 187-200
2019 年 36 巻 p. 187-200
The stability of particle suspensions, which is important in numerous industrial processes, is generally dominated by the interaction forces between the suspended particles. Understanding the interaction forces between surfaces in liquids is therefore fundamentally important in order to evaluate and control how particulates, including fluid droplets in emulsions and air bubbles in foams, behave in various systems. The invention of the surface force apparatus (SFA) enabled the direct measurement of interaction forces in liquids with molecular level resolution and it has led to remarkable progress in understanding surface forces in detail. Following the SFA, the application of atomic force microscopy (AFM) to force measurement has further extended the possibility of force measurements to a broad field of research, mainly due to the range of materials that can be employed. This review provides an overview of developments in the investigation of interaction forces between surfaces using AFM. The properties of various interaction forces, important in particle technology, revealed by the studies using AFM are described in detail.
As suspensions of particles play a role in numerous industrial processes, evaluating and controlling particle behavior in various processes is of utmost importance. Rapid developments in nanotechnology over recent years have led to the adaptation of these industrial processes to handle nanosized materials, including nanoparticles, in order to take advantage of their specific functions. Because such nanomaterials are often handled in liquids as colloidal dispersions, it has become significantly important to control the properties of nanoparticle suspensions properly and precisely, including their stability, sedimentation and rheology.
The stability of particle suspensions is generally dominated by the interaction forces between the surfaces of two opposing particles in suspension, which depend intricately on the properties of the liquid, the surfaces, solutes, and so on. Understanding of the interaction forces in liquids is therefore fundamental in many fields of science and engineering in order to evaluate and control how particulate matters (including two phase fluids such as emulsions and foams) behave in various systems.
The theoretical basis for analyzing surface forces between charged particles in aqueous solutions was provided by Derjaguin-Landau-Verwey-Overbeek (DLVO) theory in the 1940s (Derjaguin and Landau, 1941; Verwey and Overbeek, 1948), and is still used widely today. However, the detailed experimental analysis of surface forces had been impossible until the invention of the surface force apparatus (SFA) in the late 1960s. The SFA enabled the direct measurement of interaction forces both in air (Tabor and Winterton, 1968) and in liquid (Israelachvili and Tabor, 1972) with molecular level resolution, which led to remarkable progress in understanding surface forces in detail.
Following the SFA, the application of atomic force microscopy (AFM) to force measurement further extended the possibility of force measurements to new fields of research. AFM was invented as a new form of scanning probe microscope in 1986 (Binnig et al., 1986). Since its invention, the AFM has become a powerful tool in a wide range of research areas in science and engineering, including powder technology, as a technique to explore surface structures and properties. In addition to surface imaging, the introduction of the colloid probe technique (Butt, 1991; Ducker et al., 1991) enabled force measurements performed using the AFM to be compared to those performed with the SFA. In the colloid probe technique, a spherical particle is attached to the end of a probe tip and the force is measured between this particle and a flat surface, allowing quantitative evaluation of forces between macroscopic surfaces. This technique can utilize a much wider variety of materials than SFA and has therefore found applications in a wider variety of systems.
In this article, we review the development of investigating the interaction forces between surfaces, mainly focusing on studies using AFM. An overview of the various interaction forces important in particle technology is provided, as well as the principles of AFM and force measurements.
The basic composition of an AFM instrument is illustrated in Fig. 1. The probe has a very sharp tip (the typical radius of commercial tips is 2–20 nm) attached to the end of a microfabricated cantilever, usually made either of Si or Si3N4. Scanning of the probe over the sample surface is carried out with the piezoelectric scanner, which expands or shrinks in response to applied voltages. A laser beam reflected from the back of the cantilever is detected with a four-segment photodiode detector. From the location of the incident beam at the detector, vertical deflections and lateral distortions of the cantilever can be evaluated. The controller evaluates the signals from the photodetector to adjust the z-displacement of the piezo scanner, i.e. moves the sample or the probe upward and downward, to keep the feedback control parameter constant. The mapping of the resulting z-piezo movements in the x and y dimensions provides the various images of the sample surface. By using a fluid cell, operation in liquid phase is possible.
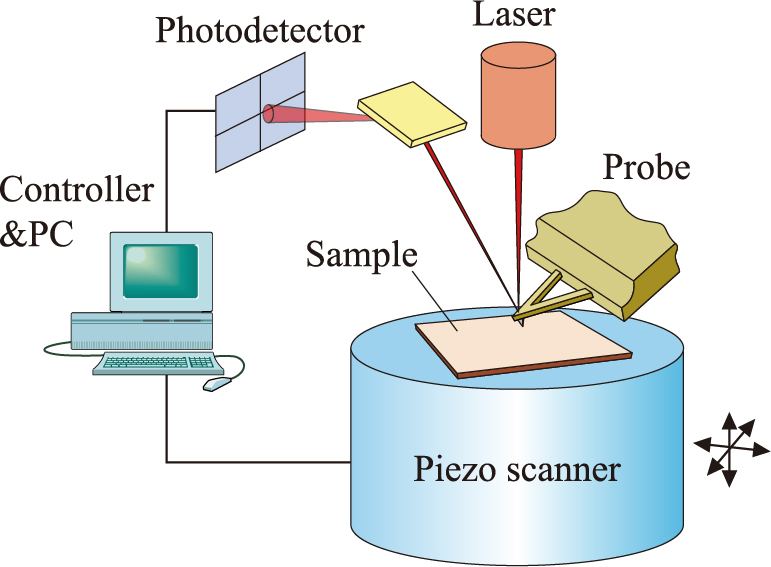
Schematic drawing of the atomic force microscope.
In order to apply the AFM to force measurements, the probe or the sample substrate is moved vertically (z-direction) to alter the distance between the probe and the surface. For the colloid probe (Fig. 2), a spherical particle typically in the diameter range of 2–40 μm is attached to the probe with epoxy glue or hot-melt epoxy resin, using a micropositioning device such as a micromanipulator whilst observing with an optical microscope or a CCD camera. During scanning, the probe-substrate interaction force is determined from the vertical deflection of the probe cantilever. Fig. 3(a) shows a schematic representation of typical deflection versus piezo translation data one can obtain by a force measurement between surfaces in an aqueous solution. In most commercial AFMs, approaching the probe to the flat substrate, bringing the surfaces into contact and retracting the probe from the substrate constitutes one measurement cycle. A positive deflection (force) value usually indicates a repulsive force. During the measurement, the deflection of the cantilever is recorded by the photodetector output voltage as a function of the relative displacement of the sample or the probe. In the case of the force in Fig. 3(a), an attractive force between the surfaces is detected. When the surfaces are in firm contact, the change in cantilever deflection equals the change in displacement as indicated by a linear region often called the “constant compliance region.”
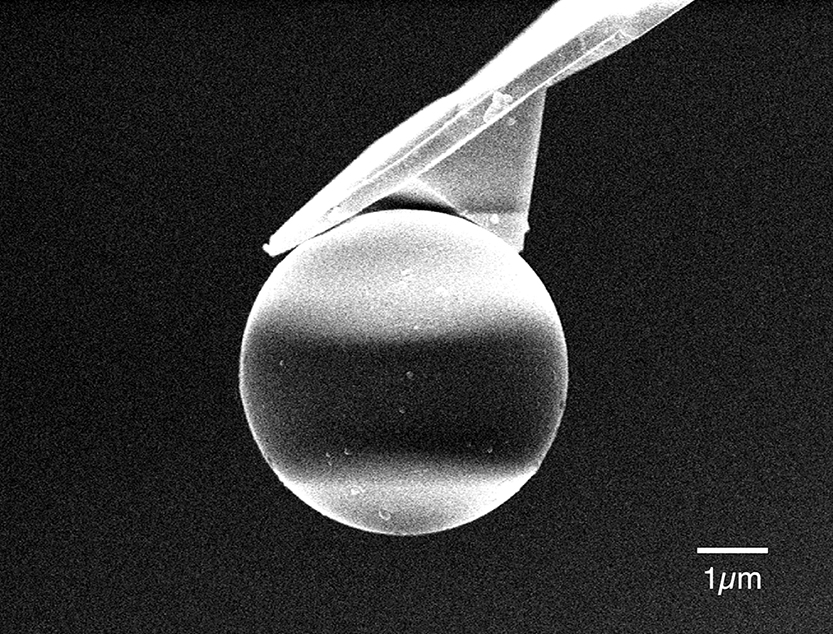
Scanning electron microscope image of a colloid probe.

Typical force data by AFM measurements. (a) The raw data and (b) the force-separation distance curve converted from (a).
Unlike the SFA, the AFM usually contains no device or system to directly measure the separation distance between the interacting surfaces. Therefore, the force versus distance relationship has to be calculated from the photodiode voltage vs. piezo displacement curve, which is obtained directly from the AFM (Ducker et al., 1991). The cantilever deflection is calculated by dividing the photodiode voltage by the slope of the constant compliance region. By setting the separation distance to zero at the constant compliance region, the separation distance at each point of the data is simply calculated by adding the cantilever deflection to the piezo displacement at each point. Then, the force-distance relationship can be obtained by multiplying the cantilever deflection with the spring constant of the cantilever k as shown in Fig. 3(b). When a colloid probe is used, the obtained force values are commonly divided by the particle radius R, because this value can be shown to be proportional to the interaction energy per unit area between parallel planes by the Derjaguin approximation.
If the attraction is a strong function of separation, the surfaces often “jump-in” to contact, due to the instability of the cantilever, which is denoted by point A and A’ in Fig. 3. This occurs when the slope of the interaction force along the separation distance exceeds the spring constant of the cantilever. As the surfaces are separated, an adhesion force may be seen between the surfaces. In this case, the cantilever usually undergoes a jump-out to zero interaction force, which is marked by point B in the figure. This is the reverse effect of the jump-in.
It should be noted that an accurate zero distance cannot be guaranteed by this method, although it is the dominant means used to determine the separation distance in AFM measurements. In particular, if there is an adsorbed layer of any kind of molecules on the surfaces and if the molecules are not excluded when the surfaces are brought into contact, a certain offset of the distance should be expected. Therefore, the distance obtained with this method should be regarded as the relative distance from the point of closest approach in the AFM force measurements and this should be kept in mind when precise distances between the surfaces need to be considered. Additionally, most surfaces have some degree of roughness and this complicates both the definition of zero separation and the practical determination of zero separation in a force measurement. (Parsons et al., 2014)
As mentioned in the Introduction, the DLVO theory provides the theoretical basis for the surface forces between charged particles in aqueous solutions. In the DLVO theory, the total interaction force between two like particles is assumed simply to be the sum of the van der Waals attraction and an electrostatic double-layer repulsion. Force measurements with the SFA and the AFM were validated by the fact that the measurements between charged surfaces in aqueous electrolyte solutions were in broad agreement with the DLVO theory (Fig. 4). This in turn confirmed that DLVO theory describes real interaction forces quite accurately.
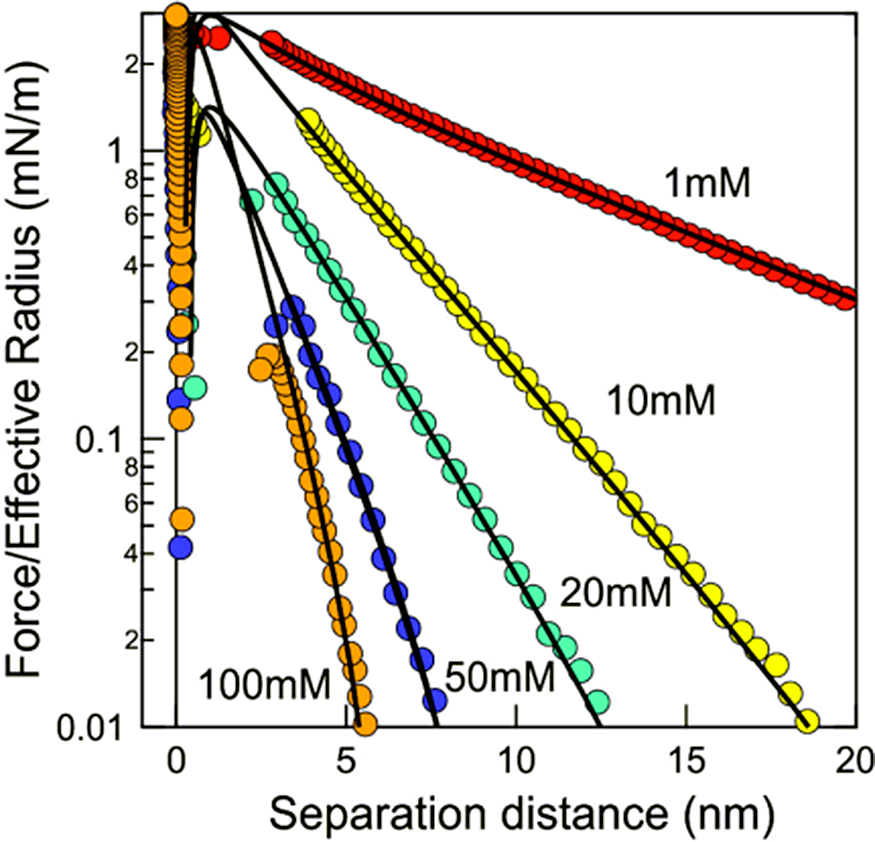
Forces between carboxyl latex particles in aqueous KCl solutions at pH4. The solid lines represent the theoretical data calculated from DLVO theory. Adopted with permission from Montes et al., 2017. Copyright (2017) John Wiley and Sons.
Since a variety of materials can be used for AFM force measurements, DLVO forces have been measured between many types of surfaces using AFM. The metals and their compounds that have been used include gold (Biggs et al., 1994), alumina (Larson et al., 1997), titania (Larson et al., 1995), zirconia (Biggs, 1995), zinc and lead sulphide (Toikka et al., 1997), iron oxide (Sander et al., 2004), tungsten (Andersson and Bergstrom, 2002) and cobalt (Andersson and Bergstrom, 2002). The forces between polymer particles have also been measured using polystyrene (Li et al., 1993), poly(etheretherketone) (Weidenhammer and Jacobasch, 1996) and Teflon (Drechsler et al., 2004). Not only the forces between identical surfaces, but those between different surfaces have also been analyzed (Larson et al., 1997; Toikka et al., 1997).
It is fundamentally important that the surfaces used in the measurement of interaction forces accurately represent the system of interest. Typically flat surfaces and spheres with an extremely low level of roughness are required in order to be able to interpret the data with confidence. In practice, however, this is not easily achieved as many materials are not available in such forms. In recent years, Atomic Layer Deposition (ALD) (George, 2010) has been applied to silicon and silica substrates to access a range of materials that are not available in a form suitable for surface force studies. This process involves a two-step gas phase chemical reaction that results in the production of a single conformal monolayer of material being grown on the surface during each cycle. By repeating the cycle a given number of times, a surface of controlled thickness can be grown on a substrate. The process can be performed with little or no increase in surface roughness, if the appropriate material and growth conditions are used (Walsh et al., 2012a). To date ALD films of alumina (Teh et al., 2010), titania (Walsh et al., 2012b), and hafnia (Eom et al., 2015) have successfully been used as substrates for force measurements.
In non-aqueous solvents, electrostatic double-layer forces are present when the dielectric constant is sufficiently high to allow the dissociation of surface groups which gives rise to a surface charge. The existence of double-layer forces have been confirmed practically in propylene carbonate (Christenson and Horn, 1983) and alcohol-water mixtures (Kanda et al., 1998). In apolar media such as hydrocarbons, the electrostatic forces can also be present when certain surfactants are added. The surfactants form reverse micelles in apolar solvents and dissolve free ions in the liquid and charge the surfaces at the same time. McNamee et al. (2004) measured the force between two silica surfaces in n-dodecane with the anionic surfactant AOT (bis(2-ethylhexyl)-sulfosuccinate) and observed electrostatic forces.
3.2 Hydration and solvation forceDevelopments in direct force measurements with the SFA and the AFM have not only provided an experimental basis for the DLVO paradigm, but have extended our understanding of the forces to those that lie outside of the DLVO paradigm. These are often called non-DLVO forces.
The hydration force is one of the most important non-DLVO forces. Although the force curves between charged surfaces in aqueous solutions measured with SFA and AFM fit well to the DLVO theory at surface separations down to several nanometers, the forces at shorter range usually deviate from the theory. This is because the DLVO theory assumes the liquid medium is a continuum, whereas the confined liquid can no longer be regarded as a continuum and the physical properties and the granularity of the liquid molecules are manifest at these small distances. Between charged surfaces in aqueous electrolyte solutions, particularly at high concentrations, monotonically repulsive forces with the range of about 1–5 nm are typically measured instead of the predicted van der Waals attraction, as indicted in Fig. 4. The repulsive force, called hydration force (Pashley, 1981), can be attributed to the energy required to remove water of hydration from surface functional groups (primary hydration) and hydrated ions from surfaces (secondary hydration) when they are brought into contact. The repulsive force can be empirically fitted with an exponential function, and its decay length is typically in the range of 0.6–1.1 nm for 1:1 electrolyte. Israelachvili and Pashley using the SFA found that the hydration force is not always monotonically repulsive but exhibits oscillations at separations below about 1.5 nm when the surfaces are molecularly smooth, like mica, and the salt concentration is low (Israelachvili and Pashley, 1983). In this case, the mean periodicity of the oscillatory force was found to be approximately equal to the diameter of a water molecule.
Hydration forces are inevitably influenced by the species of solute molecules. In experiments using SFA, the range of force between mica increased following the hydration enthalpy of the cations, in the order of Li+ ~ Na+ > Cs+ (Pashley, 1981). This tendency was also confirmed with AFM (Higashitani and Ishimura, 1997) as shown in Fig. 5. On the other hand the addition of alcohol removes the hydration repulsion (Yoon and Vivek, 1998), which is likely due to the adsorption of alcohol molecules displacing the first layer of water on the hydroxylated silica surfaces.

Forces measured between an AFM tip and a mica surface in 1 M electrolyte solutions of monovalent cations. Reprinted with permission from Higashitani and Ishimura, 1997. Copyright (1997) The Society of Chemical Engineers, Japan.
Although the hydration force is a very short-ranged force, it has a significant influence on the bulk stability of suspensions, particularly in the case of nanoparticles. It has frequently been reported that the stability of particle dispersions disagrees with theoretical predictions at high salt concentration because the coagulation rate decreases when the size of the particle is less than several hundred nanometers (Kobayashi et al., 2005), whereas in the DLVO theory, the rate should be independent of particle size. This reduction is attributed to the hydration force or related structural forces. Recently, Higashitani et al. (2017) proposed a model that can successfully predict the reduction in the coagulation rate taking into account the effect of hydration layers.
In non-aqueous solvents, the force due to the solvation and structuring of liquid molecules adjacent to surfaces that gives rise to specific interactions, are referred to as solvation forces (Horn and Israelachvili, 1981). In most cases the force is an oscillatory function with a periodicity equal to the mean molecular diameter, decaying with distance. Using AFM, the solvation force has been observed in several solvents such as OMCTS (octamethylcyclotetrasiloxane) (Han and Lindsay, 1998) and n-alcohols (Franz and Butt, 2002) (Fig. 6).
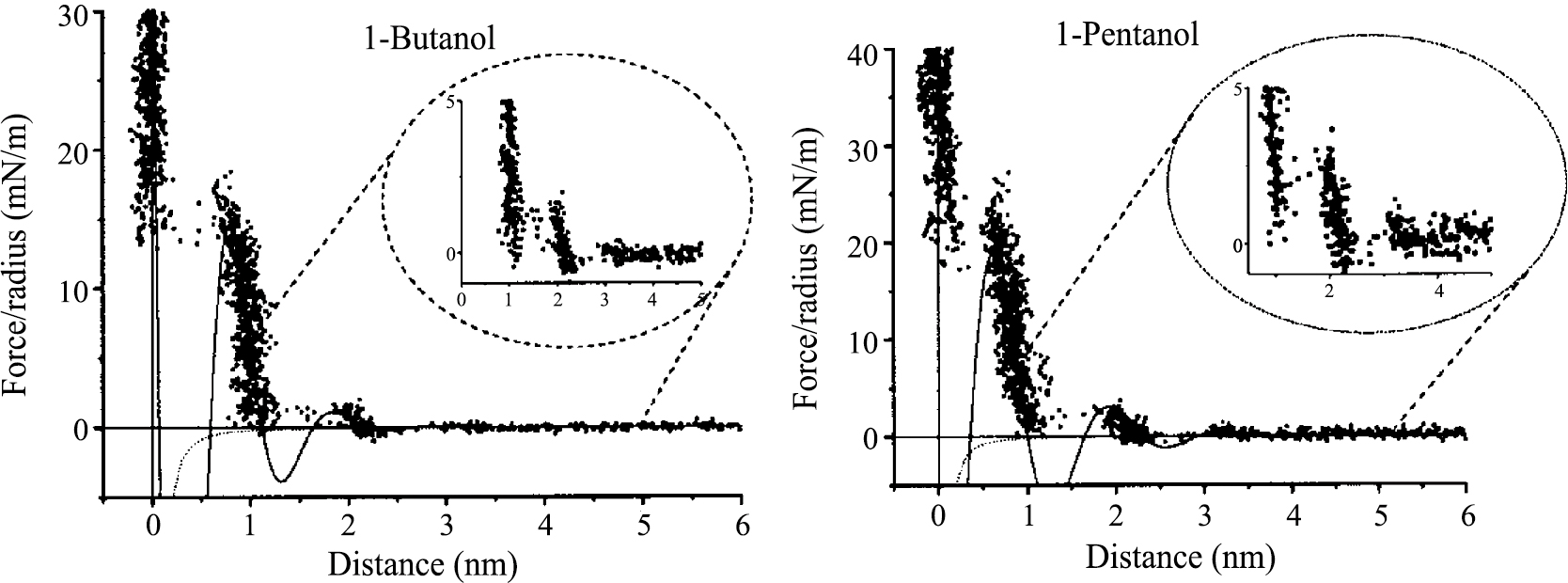
Force curves for the interaction of a silicon nitride AFM-tip with mica in 1-butanol and 1-pentanol. The forces are normalized by radius of the tip. The solid and dotted lines show the fitting with an exponentially decaying periodic function and the calculated van der Waals forces, respectively. Adapted with permission from Franz and Butt, 2002. Copyright (2002) American Chemical Society.
In various industrial processes, polymers are widely used as dispersants or flocculants to control the behavior of particle dispersions. The interactions of polymers with particle surfaces alter the interparticle forces in a manner that is dependent on the type and concentration of polymer and the interaction of the polymer with the particles, as schematically represented in Fig. 7.

Schematic drawing of the change in the interaction forces between surfaces depending on the polymer concentration. An attractive bridging force (a) is replaced with a repulsive steric force (b) in adsorbing polymer solutions when the concentration increases. The depletion force (c) acts in a solution of a nonadsorbing polymer at high concentration.
When adsorbing polymers are added to a solution with particles, aggregation occurs when the polymer concentration is low, as mentioned in the next section. If the surface coverage of the adsorbed polymer becomes sufficiently high, a repulsive interaction arises from the overlapping of the polymer molecules on opposing surfaces as shown in Fig. 7(b). This produces a repulsive force due to the unfavorable entropy of confining polymer chains between the surfaces. This repulsive force is well known as steric repulsion and is the controlling force when polymers act as dispersants in liquids. AFM measurements of steric repulsion have mainly been conducted between silica surfaces with polymers adsorbed from solution (Musoke and Luckham, 2004; McLean et al., 2005) or polymers grafted onto silica (McNamee et al., 2007).
Currently there is no simple, comprehensive theory available to describe experimentally measured steric forces, as steric forces are complex and are influenced significantly by many factors. The magnitude of the force between surfaces with polymers depends on the quantity and density of polymer on each surface and on whether they are physically adsorbed or irreversibly grafted onto the surfaces. The quality of the solvent also affects the force. For two brush-bearing surfaces, the Alexander-de Gennes (AdG) theory is usually used to approximate the steric force between them, while the theory has a limited validity for the steric force between physisorbed surfaces. McLean et al. (2005) reported that the Milner, Witten, and Cates (MWC) model (Milner et al., 1988) gives a better fitting than the AdG theory for the interaction between hydrophobic surfaces with adsorbed triblock copolymer layers, presumably because the segment density profile on the adsorbed polymer on the surfaces is in line with the assumptions of the MWC model. Further, it is theoretically and experimentally difficult to decouple steric forces from hydrodynamic forces associated with the expulsion of solvent during compression of a polymer brush or film (Wu et al., 2018).
The steric force also acts between surfaces with adsorbed aggregate structures, such as micelles. The adsorption of charged surfactant micelles onto the surfaces gives strong steric repulsion at short range, along with electrostatic repulsion (Stiernstedt et al., 2005). Between silica surfaces in solutions of nonionic poly(oxyethylene) surfactants, a strong repulsive force was also observed at short range, at concentrations above the critical micelle concentration, due to the formation of surfactant bilayers (Rutland and Senden, 1993).
3.4 Bridging of polymers and depletion forcesAs mentioned in the previous section, when adsorbing polymers are added to a dispersion of particles, aggregation occurs when the polymer concentration is low, as drawn in Fig. 7(a). In this case the low surface coverage of the polymers allows the bridging of polymer molecules between particle surfaces, resulting in an attractive force evident at large separation distance. With the AFM, this attractive bridging force, with a range of several 10’s of nanometers, was observed by Biggs (1995) between zirconia surfaces in polyacrylamide solutions and by Zhou et al. (2008) for cationic polymers between silica surface.
While polymers adsorbed at high concentration give rise to steric repulsion, the interaction is attractive in nonadsorbing polymer solutions at high concentration, as shown in Fig. 7(c). Empirical knowledge of the ability of nonadsorbing polymers to promote particle flocculation has been long known. During the 1950s, Asakura and Oosawa presented a theory for an attractive force, referred to as a depletion force, as an explanation for this flocculation (Asakura and Oosawa, 1954). When solid surfaces are in a solution of a nonadsorbing polymer at high concentration, the polymer molecules are excluded from the region between the surfaces, when the distance between them is less than the effective diameter of the polymer. This reduces the osmotic pressure between the surfaces relative to the bulk solution, resulting in an attractive depletion force.
The existence of the depletion force was experimentally confirmed between stearylated silica surfaces in polydimethylsiloxane solutions in cyclohexane (Milling and Biggs, 1995). The depletion forces were observed not only in polymer solutions but in solutions containing surfactant micelles (Richetti and Kékicheff, 1992) and solid nanoparticles (Sharma and Walz, 1996), indicating that the depletion phenomenon is common in solutions containing nonadsorbing particulate matters at high concentration.
3.5 Hydrophobic attractionIt had been known empirically that there is a strong attractive force between hydrophobic particles in aqueous solutions as they aggregate quite rapidly. The first direct evidence that the force is of greater magnitude than the van der Waals force was obtained between mica surfaces bearing adsorbed cationic surfactant, by Israelachvili and Pashley with the SFA (Israelachvili and Pashley, 1982). Since then countless studies have been performed to elucidate the nature of the hydrophobic attraction. The origin of the hydrophobic attraction, however, was much disputed because the experimentally observed forces have sometimes shown an inconceivably long-range, that reaches up to several hundreds of nanometers (Kurihara and Kunitake, 1992), which no conventional thermodynamics can explain. In addition, the fact that the observed forces had a variety of ranges and magnitudes depending on the systems used (Christenson and Claesson, 2001) precluded a single theory from describing all the experimental results.
Gradually the contribution of numerous studies investigating the hydrophobic attraction, has revealed the origin of the measured force and multiple phenomena have been found to apply depending on the system. Bridging of nanobubbles is now recognized as the main cause of the very long-range forces. This mainly occurs when the surfaces are highly hydrophobic and robust, such as chemisorption of materials that produce hydrocarbon layers, as an example of the force data is shown in Fig. 8(a). The existence of stable nanobubbles on surfaces in contact with an aqueous solution was first predicted in the mid-1990s (Parker et al., 1994) and was later experimentally confirmed by AFM observations (Ishida, et al., 2000a) (Fig. 8(b)) in relation to the hydrophobic attraction (Ishida et al., 2000b). On the other hand, when hydrophobic surfaces are prepared by physical adsorption of surfactants or amphiphiles, electrostatic forces can arise as they tend to form domains of aggregates on the surfaces (Zhang et al., 2005). Such aggregates can form as the surfaces approach (Meyer et al., 2005). Domains of these aggregates create patches with different charge properties. Upon interaction it is favorable for oppositely charged regions to align and this results in a long-range electrostatic attractive force that is dependent on the ionic strength of the solution.

Typical approaching and retracting force curves between silica surfaces hydrophobized with octadecyltrichlorosilane (OTS) measured in water (a) and a tapping-mode AFM image (3 × 3 μm2) of the substrate hydrophobized with OTS obtained in water (b). The steps that appear in the approaching and retracting force curves in (a) are considered to represent the force changes when a bridging of nanobubbles between the surfaces forms and disappears. The nanobubbles on the surface are observed as domain-like structures (bright regions) in the image in (b).
Recent studies on the hydrophobic attraction are focusing on the “pure” component of the force, because the forces arising from the bubbles or charge domains are sometimes not regarded as that produced by the surface hydrophobicity itself. Indeed, the hydrophobic attraction has been found between highly hydrophobic surfaces in the absence of nanobubbles or electrostatic attraction (Ishida et al., 2012), as shown in Fig. 9. The general consensus thus far seems to be that such “pure” hydrophobic attraction has a short-range of less than 10–15 nm for all types of hydrophobic surfaces (Meyer et al., 2005). Although the origin of the force is still under intense scrutiny, this attraction seems to have an exponential form that decays with the surface separation. The experimentally obtained value of the decay length often lies in the range of 0.3–2.0 nm (Donaldson et al., 2015). On the other hand, Tabor et al. (2013) measured the forces between fluorocarbon oil droplets with the refractive index matched to that of solution, in order to minimize the effect of the van der Waals attraction. They measured D0 value to be 0.3 nm, shorter than that for solids. This difference in the decay length implies that the nature of the pure hydrophobic force could also vary depending on the system. Ishida et al. (2018) measured the interaction forces between silica surfaces modified to different degrees of hydrophobicity and found one surface is highly hydrophobic, this promotes the hydrophobic attraction such that it is observed even against a mildly hydrophobic surface. In this case, the contact angle of the other surface dominates the range and strength of the force and nanoscopic properties of the molecules on the surface play only a minor role. However, despite these contributions the mechanism responsible for this “pure” hydrophobic attraction remains unresolved and further studies are still necessary to reveal the origin of the force.
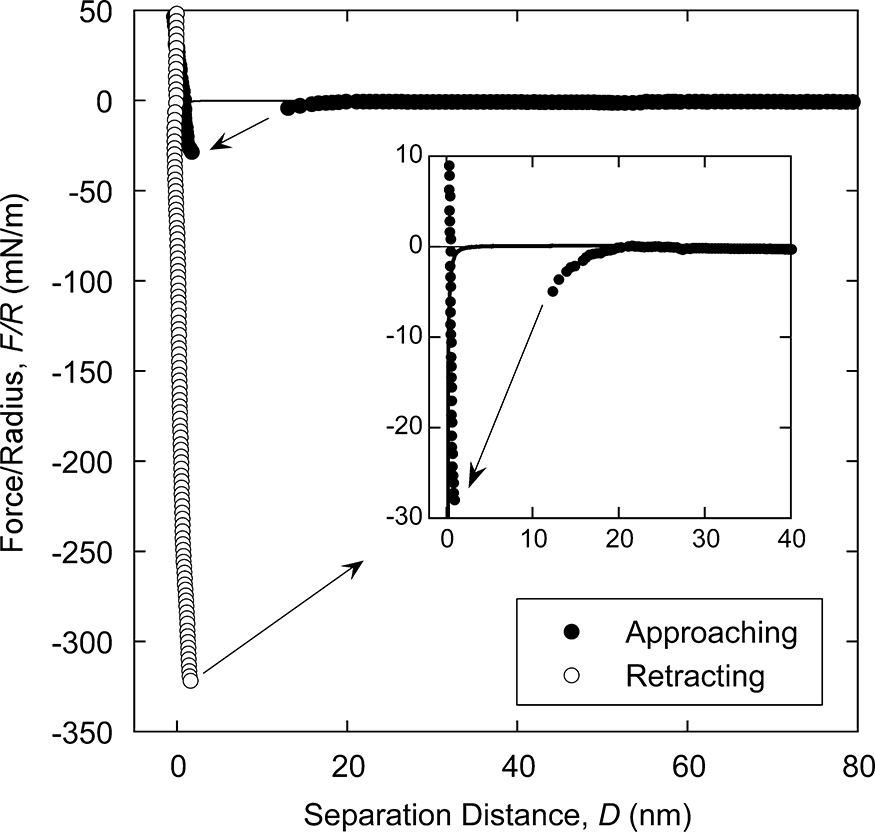
Approaching and separating force curves between hydrophobic (OTS-coated) silica surfaces in 1 mM NaNO3 solution obtained after the nanobubble-removing process. The inset shows a close-up plot of the short-range region of the approaching force. The solid line shows the calculated van der Waals attraction.
Capillary condensation of water vapor and bridging of condensed water films between surfaces is a well-established origin of a long-range and strongly attractive force between particles in the gas phase. Similarly, capillary-induced phase separation and bridging in a liquid phase often produces strong and complex interaction forces.
In several different cases it has been observed that confinement between surfaces has induced a phase separation, resulting in an attractive force. The best example of this would be water in nonpolar (water-insoluble) solvents. A trace amount of water present in cyclohexane was found to give rise to an extraordinary long-range force, the range of which was up to 250 nm, between silica surfaces (Kanda et al., 2001), as shown in Fig. 10. This force originates from the capillary condensation of water between the surfaces, due to its higher affinity with the silica surfaces than cyclohexane. The Laplace pressure associated with a bridge of the condensed water phase causes a long-range attraction. Similar phenomenon has also been observed in alcohol-cyclohexane mixtures (Mizukami et al., 2002), in which case the alcohol molecules are thought to form a network structure. Lee et al. (2011) found such capillary condensation occurs even in a mixture of miscible solvents. They found a long-range attractive force between a silica particle and a glass plate in mixtures of water and N-methyl-2-pyrrolidone (NMP), which are strongly heteroassociating liquids, when the NMP concentration range is within 30–50 vol%. In this case, it was also suggested that the bridging of macrocluster layers of water and NMP formed adjacent to hydrophilic surfaces.

Interaction forces between silica surfaces in cyclohexane with various amount of water. In the plot, φw denotes the mixing ratio of water-saturated cyclohexane (contains 68 ppm of water) to dried cyclohexane. Reprinted with permission from Kanda et al., 2001. Copyright (2002) The Society of Powder Technology, Japan.
Attractive forces also arise by the capillary condensation of solutes between surfaces. Solutions of polymer mixtures particularly have a strong tendency to phase separate and form two phases. Wennerström et al. (1998) observed a very long-range attractive force between mica surfaces in mixed aqueous systems of dextran and poly(ethyleneoxide) (PEO) using SFA. Further, Sprakel et al. (2007) found capillary condensation of polymer molecules from aqueous solutions, measuring a long-range force between hydrophobic surfaces in solutions of alkylchain-terminated PEO. They pointed out that in this case the interfacial tensions between the capillary bridge and the coexisting bulk phase is extremely low, on the order of 10 μN/m. Long range attractive forces between alumina surfaces in the presence of muconic acid were also attributed to capillary bridging due to the low solubility of the munconic acid (Teh et al., 2010).
3.7 Force between fluid interfacesInteractions of particles with fluid interfaces such as air bubbles and oil droplets are found in many industrial processes, such as froth flotation. The interactions of a solid surface with fluid interfaces, therefore, should be as important as particle–particle interactions. Evaluation of the interactions between a particle and a bubble or oil droplet with the AFM or with AFM related setups, however, has not been as widely adopted as measurement of the solid-solid interactions. Whilst the measurements with the AFM can be readily performed by using the colloid probe technique in which the probe interacts with a small bubble or oil droplet attached to a hydrophobic plate in a liquid cell, interpretation of the obtained forces is often quite complex because of the deformation of fluid surfaces. The colloid probe, if hydrophobic, can even penetrate into the bubble or oil droplet.
Nevertheless, understanding of the interaction force between particles and fluid interfaces has progressed due to several studies. Fielden et al. (1996) confirmed that a monotonically repulsive force acted between a hydrophilic silica particle and an air bubble. The repulsive force fits to DLVO theory, indicating the water–gas interface is negatively charged at neutral pH as reported in previous measurements of bubble zeta potential (Li and Somasundaran, 1991).
The interactions of fluids with hydrophobic particles are more complicated to analyze because the attraction between a particle and an interface of the fluid, and the engulfment of the particle into the fluid happens almost simultaneously. In this case, the measured forces usually only reveal a single long-range jump and most of the detailed information on the attraction between the surface is lost. Ishida (2007) measured the forces between a hydrophobized silica particle and a bubble and estimated the separation of the surfaces just prior to the particle jumping in and being engulfed in the bubble by fitting the repulsive force before the jump to the DVLO theory. The thickness of the water film between the particle and the bubble surface when it ruptured was estimated to be 10–15 nm. Interestingly, this thickness is very close to the distance of the hydrophobic attraction, which is mentioned in the previous section.
The interaction between a particle and an oil droplet is similar to that between a particle and a bubble. As oil surfaces are usually negatively charged, a repulsive interaction has been found between a silica particle and a decane droplet with a decay length equal to the Debye length (Hartley et al., 1999).
Recently, an Australian group has successfully developed methods to measure and analyze forces between two fluid interfaces in solution (Dagastine et al., 2006; Clasohm et al., 2007; Vakarelski et al., 2010), such as bubble-bubble and oil-oil, as shown in Fig. 11. They attached a small bubble or an oil droplet on the top of a specially microfabricated cantilever and measured the forces of interaction with another bubble/oil droplet deposited on a hydrophobic plate. The separation distance of the surfaces and the deformation of the bubbles was calculated using mathematical models combining the surface forces, hydrodynamics and the deformation of the surfaces. One of the important findings they provided in the series of the experiments is that the coalescence of bubbles and oil droplets occurs within the paradigm of the DLVO force–no additional force was evident. This was surprising as a hydrophobic attraction had been expected based on earlier experiments on solid hydrophobic surfaces. Instead, hydrodynamic forces were influential, deforming the surfaces and inducing water drainage during the thinning of the water film between the surfaces.

The approaching (open symbols) and retracting (filled symbols) interaction force versus piezo drive motion ΔX between two decane droplets in 0.1 mM SDS solution in water measured at different probe velocities (green circles, 2 μm/s; blue triangles, 9.3 μm/s; red diamonds, 28 μm/s). The solid lines are the calculated force curves from a comprehensive model of the dynamic droplet interactions. Translated from Dagastine et al., 2006 with permission from AAAS.
In addition to “static” interactions described above, the hydrodynamic force is certainly an important issue in processing particles during stirring, separation, and transportation. Most AFM studies related to the hydrodynamic force seem to have focused on the boundary slip condition of Newtonian liquids over a solid wall. In classical fluid mechanics, the assumption was made that when a liquid flows over a solid surface, the liquid molecules adjacent to the solid have the same velocity as the solid (i.e. stationary relative to the solid), this is called the non-slip boundary condition. The boundary slip of liquid along a solid surface, however, has been observed at the micro-and nanoscale under certain conditions by sensitive experiments. The degree of boundary slip is characterized by the slip length, which is defined as the distance behind the interface at which the liquid velocity extrapolates to zero.
The first utilization of the AFM to measure the boundary slip in aqueous solutions was made by Craig et al (2001). They used the colloid probe technique to measure the hydrodynamic drag force between gold-coated silica and mica with a partially hydrophobic coating, in aqueous sucrose solutions. They observed slip occurring with a varying slip length of up to ~20 nm, depending on the liquid viscosity and the shear rate. As the result of numerous studies conducted following this report, it is now generally accepted that slip occurs for liquids on lyophobic surfaces, although a broad range of the observed slip length depends on the experimental conditions. On the other hand, when the surfaces are completely wetted and smooth, slip is not likely to occur (Maali et al., 2009), though surface roughness could induce slip (Bonaccurso et al., 2002).
3.9 Friction forceIn circumstances where the flow of solutions with relatively high particle loadings occurs, not only normal forces but frictional forces between particles are important. In addition, the frictional forces play an important role in emerging technologies such as chemical mechanical planarization (CMP) and microelectromechanical systems (MEMS). As such, understanding of frictional phenomena at a nanoscale level has become one of the critical topics in various fields of technology.
The measurement of friction by AFM can be conducted by scanning a probe over a surface with a constant normal load and measuring the twisting of the cantilever using the photodiode output in the lateral direction. From the magnitude of the twist, the lateral force can be determined as a function of the normal load. Biggs et al. (2000) was the first to apply the colloid probe technique for friction measurements. They observed a difference in the friction properties depending on the surface hydrophobicity.
Higashitani’s group has extensively investigated the friction between silica surfaces in aqueous electrolyte solutions. They found that the presence of salt ions has shown better lubrication between silica surfaces than pure water and the degree of lubrication has been found to follow the order of hydration of cations (Li+ > Na+ > Cs+) (Donose et al., 2005), as shown in Fig. 12. This can be attributed to the lateral mobility of the water molecule in the hydration shell of adsorbed cations. The friction was found to increase linearly with the applied load without any dependence on pH in the range of pH 3.6 to 8.6, whereas the friction becomes extremely small and the dependence on the applied load became nonlinear at higher pH (Taran et al., 2007). They suggested that this transition is caused by the development of a gel layer composed of polymer-like segments of silicic acid anchored on the surface at high pH.
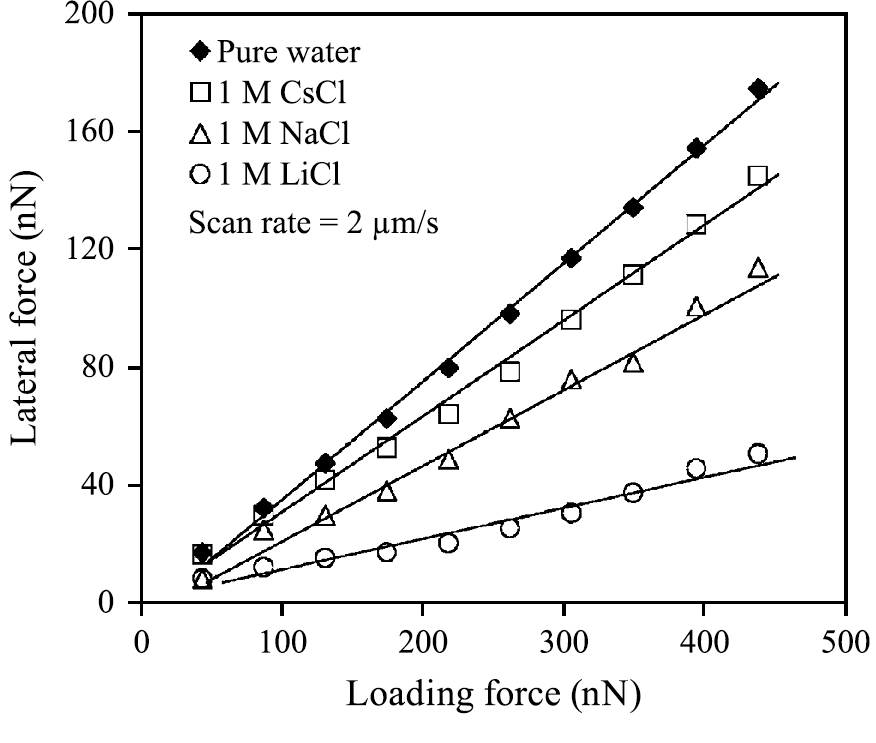
Dependence of friction force on the applied load measured between a silica particle and a silica wafer in pure water and CsCl, NaCl, or LiCl solutions of 1 M. Adapted with permission from Donose et al., 2005. Copyright (2005) American Chemical Society.
Adsorption of surfactant also reduces the friction coefficient. Vakarelski et al. (2004) reported that the friction between silica surfaces in a cationic surfactant solution reduced with increasing concentration and became constant at concentrations higher than the CMC. An adsorbed layer of the weak polyelectrolyte (Notley et al., 2003) and hydrophilic polymer (Li et al., 2011) also reduced the friction between surfaces, implying that any form of hydrated layer will play an important role in friction reduction.
This review has presented an overview of how AFM has been employed for investigating the interaction forces between surfaces and interfaces. The studies introduced herein clearly show that AFM is arguably one of the best tools for gaining direct understanding of how the interaction forces change depending on the properties of the liquid, the surfaces, and solutes down to the nanometer scale.
There are some challenges in AFM force measurements that need to be resolved to allow the application of this technique to an even broader range of research problems. One of the issues is the application of the colloid probe method to interactions between nanoparticles. The rapid progress of nanotechnology has led to a demand for measurement of the interactions between nanoparticles directly. As the behavior of the particles is affected by the size and/or the shape of the particle, it is unclear whether the force data for spherical particles with a diameter of several micrometers could be directly applied to analyze the behavior of real nanoparticles. As nanoparticles are too small to be observed optically, effective methods are certainly necessary to attach a nanosized particle stably on the tip of an AFM. Even then for small nanoparticles the presence of tip itself will alter the interaction forces.
Correlation between the interaction forces measured by AFM and the behavior of real suspensions is another important challenge in the field of particle technology. The complicated behavior of concentrated slurries in particular, has scarcely been described in terms of particle interactions. Understanding of the effect of interaction forces on the precise rheological properties of suspensions remains limited. This requires that the surface forces measured between two surfaces be incorporated in understanding how this influences the larger scale structure of the dispersion and the forces between many particles. Further improvements in AFM force measurements in combination with other experimental methods and with other approaches such as computer simulation is expected to achieve a more comprehensive understanding of the behavior of particulate matters in liquids in the foreseeable future.
N.I. acknowledges financial support by KAKENHI (Grants 25420803 and 15KK0238) from the Japan Society for the Promotion of Science (JSPS) and by a research grant from Hosokawa Particle Technology Foundation. Partial financial support by Adaptable and Seamless Technology Transfer Program, Target-driven Research (A-STEP) Stage I from the Japan Science and Technology Agency (JST) is also gratefully acknowledged. V.S.J.C. acknowledges the support of the Australian Research Council (DP140102371).
Naoyuki Ishida
Naoyuki Ishida is an Associate Professor in the Department of Applied Chemistry and Biotechnology at Okayama University. After completing his Ph.D. degree at Kyoto University in 2000, he spent 12 years at the National Institute of Advanced Industrial Science and Technology (AIST) as a researcher, and then started an academic carrier at Okayama University in 2012. His research interests include the direct measurement of surface forces in liquids, the measurement of interaction forces between biomolecules, and the characterization of stimuli-responsive polymers.
Vincent S. J. Craig
Prof. Vincent Craig leads the colloids group in the Department of Applied Mathematics at the Australian National University. He completed both his B.Sc. (Honours in Chemistry in 1992) and Ph.D. degrees (jointly in Applied Maths and Chemistry in 1997) at the ANU before postdoctoral positions at UC Davis, California and the University of Newcastle, NSW.
He was awarded an ARC Postdoctoral fellowship in 1998, an ARC Research Fellowship in 2001 and an ARC Future Fellowship in 2009. His research interests include the measurement of surface forces both quasi-static and dynamic, adsorption of surfactants and polymers, specific ion effects and nanobubbles.