Abstract
Lithium-ion batteries (LIBs) provide the largest source of electrical energy storage today. This paper covers the use of comminution processes and, thus, crushers and mills for particle breakage and dispersing, as well as classifiers for particle separation within the process chain, from the raw material to the final lithium battery cell and its recycling at end of life. First of all, the raw materials for the active material production have to be produced either by processing primary raw materials, or by recycling the spent lithium batteries. The end-of-life battery cells have to be shredded, the materials separated and then milled in order to achieve the so-called black mass, which provides a secondary material source with very valuable components. Using these materials for the synthesis of the cathode active materials, milling has to be applied in different stages. The natural graphite, increasingly used as anode material, has to be designed in mills and classifiers for achieving targeted properties. Nanosized silicon is produced by nanomilling using stirred media mills as a primary option. Conductive additives for LIBs, like carbon black, have to be dispersed in a solvent with machines like planetary mixers, extruders or stirred media mills. In the future, mechanochemical synthesis of solid electrolytes will especially require additional application of comminution processes.
1. Introduction
The most popular energy storage system today is the lithium-ion battery (LIB). LIBs offer superior performance in terms of energy density (Wh/L) and specific energy (Wh/kg), as well as lifetime and cycling stability. Moreover, due to their mass production, their costs decrease continuously and, due to increasing application knowledge, the safety is steadily improving. Regarding LIB cell costs, about 75 % is attributed to the materials used for the cell production (Kwade et al., 2018). A schematic presentation of an electrochemical basic unit consisting of cathode, separator and anode, as well as electrolyte, is shown in Fig. 1 left. Both electrodes consist of particles which are bound to each other and to a current collector by a binder. As active material on the cathode side, particles consisting of lithium transition metal oxides (e.g. NMC, NCA, LMNO) or a lithium metal phosphate (e.g. LFP) are used together with carbon based conductive additives like carbon black, carbon nano tubes (CNT) and/or fine graphite particles. On the anode side, as a rule, natural or synthetic graphite particles with tailored surface functionalizations are employed together with small amounts of carbon-based conductive additives. Recently, the specific capacity of the anodes has been increased by adding smaller amounts of silicon, which has about a 10 times higher specific capacity than graphite, despite possessing severe cycling stability challenges due to extreme volume changes during cycling.

In addition to the development of improved active materials, comprehensive research is being conducted on the development of solid electrolytes for the so-called All-Solid-State Batteries (ASSB) as next generation lithium battery technology (Janek and Zeier, 2016). As depicted in Fig. 1 right, such ASSB cells have a solid electrolyte acting as ionic conductor within the cathode, as well as a separator layer between anode and cathode. Solid electrolytes can be sulfide, polymer and/or oxide based, among others, and are produced by mechanochemical processes. Lithium based anodes can be employed in an ASSB, avoiding, or at least strongly minimizing, lithium dendrite growth, as well as significantly increasing the energy density.
Another promising lithium battery cell type is the Lithium-Sulfur-Battery (LiS), which has the potential to achieve very high specific capacities, which is especially important for aerospace applications or application within trucks (Zhao et al., 2020). Similar to ASSB, these cells have a lithium-based anode and a cathode based on a sulfur-carbon composite material. Both liquid electrolyte and solid electrolytes can be employed.
Today the different materials have to be produced from primary resources mainly by mining and subsequent mineral processing. For the metals lithium, nickel, cobalt, manganese, aluminum and copper, as well as for graphite, almost 3 million tons in 2025 (with approx. 0.5 million tons of nickel and graphite) and about 7 million tons will be required in 2030 (with approx. 1 million tons of nickel and graphite) overall according to the Electric Vehicle Outlook 2018 of Bloomberg New Energy Finance (Bloomberg NEF, 2018). Therefore, the conservation of primary material resources, and interdependence on individual primary resources and raw material producers, is an important task for the coming years. Moreover, the actual development challenges are the improvement of the fast-charging behavior and the CO2-footprint of the LIB. In order to reduce the CO2-footprint and to become independent of the primary raw material resources, the buildup and establishment of a circular economy, especially circular production of LIB, is a major task for the near future. Within the circular production, the End-of-Life (EoL) battery systems have to be dismantled and the components have to be fed to a recycling process, resulting in secondary raw materials that are used to produce new battery active materials, especially cathode active materials. The overall recycling process can consist of mechanical (especially shredding, milling), classification and sorting, thermal, hydrometallurgical and/or pyrometallurgical process steps (Doose et al., 2021b). According to existing or proposed regulations in the different regions of the world (e.g. newly proposed EU directive), batteries must be recycled at high recycling rates, especially for reuse of the transition metals as Ni, Co and Cu, with up to 95 % recovery, as well as for Li and the overall battery system with at least 70 % recovery. A schematic outline of the circular production of lithium batteries, especially lithium-ion batteries, is shown in Fig. 2.
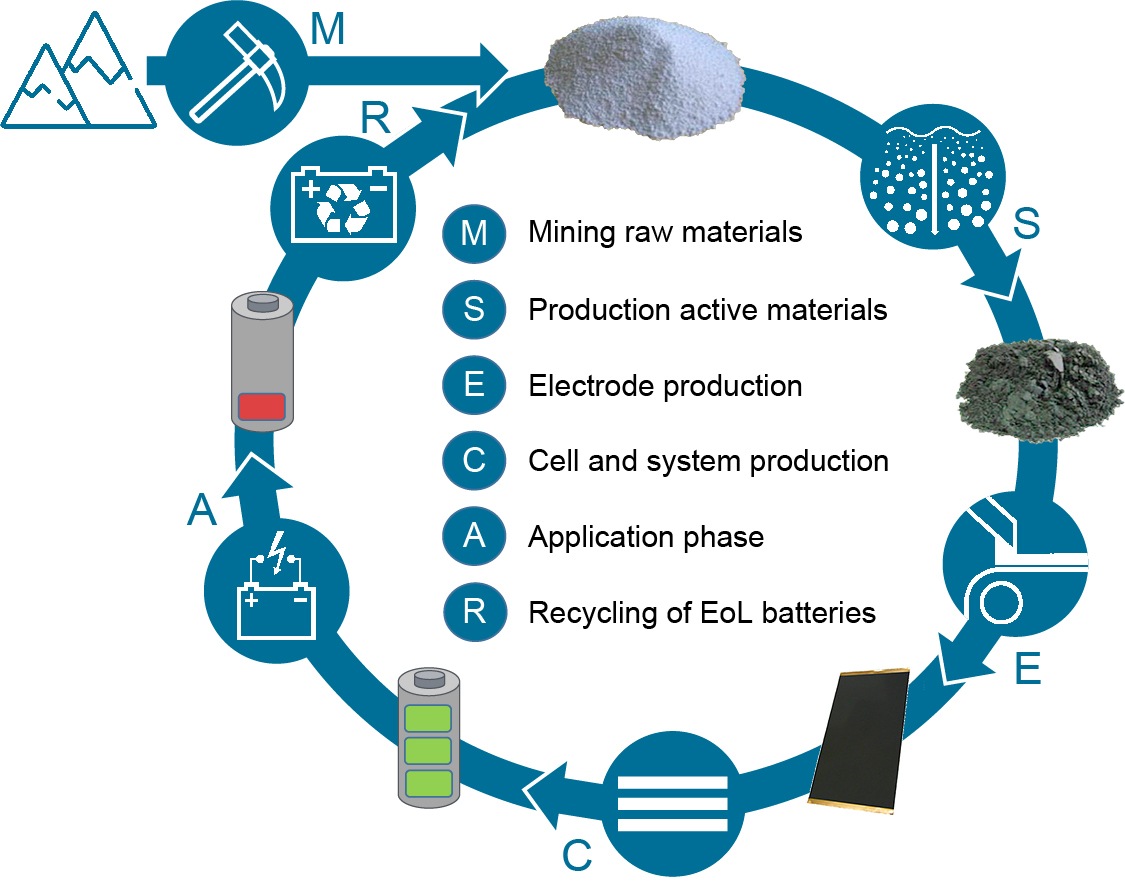
The primary, and increasingly the secondary, raw materials are used as the basis for the production/synthesis of the cathode and anode active materials. Within the production of these particulate active materials, milling and classification processes play a major role for tailoring the particle size distributions and particle shapes, as well as dispersing calcinated materials. The particle size distributions and particle shapes are important for the subsequent electrochemical performance and cycling stability of the LIB cells.
For the production of the anodes and cathodes, the different components (as shown in Fig. 1) have to be well dispersed in a solvent by using mixing and milling devices. The resulting microstructure of the carbon-based conductive additives is decisive for the future cell performance. After production, the electrodes are assembled together with the separator to form the battery cell, as described in detail for example in the literature (Kwade et al., 2018). The cells can be cylindrical, prismatic or pouch types, and are lastly filled with electrolyte, formatted, aged and closed to be shipped for integration into a battery system for application in electromobility, stationary storage or power tools. After the application phase, usually lasting more than ten years, the lithium-ion batteries reach their end of life and have to be shipped to a dismantling and recycling facility.
Finally, besides the critical discussion and further presentation of the perspective of milling, dispersing and classification processes for today’s LIB based on liquid electrolytes, an outlook of milling and classification processes for the production of ASSB will be given. The synthesis of solid electrolytes is often achieved through mechanochemical synthesis within planetary, vibration or agitated ball mills. Moreover, mills are often employed for the preparation of the composite materials for the cathode. Another possible application is the production of composite materials for LiS battery cells, which have high potential for usage in aviation, due to their low weight in relation to their storage capacity.
2. Raw material supply
Today, the raw materials for the cathode active material production, mainly ores with transition metals, and for the anode active material production, i.e. natural graphite and synthetic graphite, as well as silicon, are extracted from primary natural resources. However, the electromobility and energy turnaround can only be successful if the materials used for the production of the energy storage devices underly a circular economy: i.e. closed material cycles. Therefore, besides primary resources, the use of EoL batteries as secondary material source is growing in importance. Within the processing of both primary and secondary raw materials, comminution, classification and sorting processes play a major role.
2.1 Processing of primary raw materials
Mineral processing is the link between mining and chemical beneficiation of valuable elements, like lithium, for battery applications. The valuable minerals upon which our industrialized society depends have been formed and or laid down in host rock, from which they need to be extracted. The minerals are embedded and dispersed in many forms and structures, presenting a wide range of requirements for their recovery from different ore bodies. Most valuable metals occur naturally in the form of their oxides, sulfides, silicates, carbonates or similar. Extractive processing usually involves the conversion of the mineral into the valuable metal, or for lithium to a soluble component like LiCO3 (Amarante et al., 1999; Talens Peiró et al., 2013; Lee, 2015). Mineral processing is responsible for concentrating the raw mined rock, which generally contains the valuable minerals in concentrations from a few percent down to parts per million, to a concentrate stream with 10 % to 40 % valuables that is sent for refining to the end metal product. In order to concentrate the minerals, they have to be mechanically separated from the host rock to be exposed to a recovery process. The exposure should be sufficient to differentiate particles that contain the valuable minerals from those that are barren, or have an extremely low grade of valuables. The recovery process can be physical (density, magnetic susceptibility), based on surface chemistry (flotation), or exposure to a lixiviant used to dissolve the minerals (leaching), each requiring a different degree of mineral exposure. The size to which the rock needs to be reduced depends upon the mineral association and recovery process. The process of rock breakage for liberation of the minerals is known as comminution.
Predictions that mineral processing would become less important in favor of hydro- and pyrometallurgy are only true for a few special cases (Moskvitch, 2014). For most ores, including lithium containing pegmatites, mechanical breakage and concentration creates reactive surfaces suited to hydrometallurgical recovery of the valuable components (Baláž, 2003). For lithium ores, concentration is followed by either calcination or roasting, and then a leaching step. Both are often followed by a carbonate precipitation (Barbosa et al., 2014; Amer, 2008; Sitando and Crouse, 2012; Yan et al., 2012).
In order to reduce the particle size by about a thousand-fold, a number of progressive breakage stages are required. Blasting of in-situ rock, followed by primary crushing, provides a minus 200 mm feed of rock. This can be stage-crushed to minus 12 mm for grinding in tumbling ball mills that use steel grinding media, and produce products in the range of 80 % passing (P80) 60 μm up to 80 % passing 1 mm. Large semi-autogenous grinding (SAG) mills that contain a mixture of rock and steel balls as grinding media are common for large ore bodies of low grade. SAG mills are fed direct from the primary crusher, have up to 24 MW power draw, and process 1500 to 3500 tons per hour (tph) of ore. They are generally followed by one to three ball mills. For a required grind size finer than 60 μm, stirred mills are generally used as the final grinding stage. These provide a lower energy use for fine grinding, and the higher energy density reduces the footprint of the equipment (Palaniandy et al., 2015; Taylor et al., 2020).
Most commonly, hydrocyclones are used to classify the product by size prior to the recovery process. However, these are intrinsically inefficient and allow coarse oversize to be passed on, and fine final product to return to the mill and be over-ground. Additionally, the milling process is non-selective, resulting in overgrinding of material as is passing along a mill. More extensive descriptions of the comminution process can be found in works such as those of Wills’ Mineral Processing Technology (Wills and Napier-Munn, 2006).
Comminution is exceedingly energy-intensive, using 2–3 % of the electrical energy consumed worldwide energy and around 40 % of energy in mining (Ballantyne and Powell, 2014). The energy requirement rises almost exponentially with finer grinding, but linearly with the production of fine product (Ballantyne et al., 2015), emphasizing the need to improve processing at the fine end (minus 500 μm), or reduce the need to produce material below this size. Due to this energy intensity, the need to drive down the energy cost is a significant factor in reducing the environmental cost of producing metals. This has led to many ongoing process improvements designed to reduce energy consumption.
Alternate circuits that include high pressure grinding rolls (HPGR) (Schönert, 1988) are becoming more common for hard-rock applications, as they offer a reduction in energy use, but they still need to be fed by conventional crushers and require ball mills to produce the final grind size, compromising the overall energy savings. Selective breakage of minerals along the grain boundaries has long been a pathway pursued to reduce energy and improve recoveries. It has been met with limited success thus far, such as in the application of microwave pretreatment (Kingman, 2006), or electrical fragmentation (Parker et al., 2015), but remains an important area of research.
In light of the high energy use and low efficiency of breakage and selection of minerals, a major research thrust is captured in the work of Powell (2019): “The objective of industrial comminution processes is to conduct the minimum degree of breakage required to permit recovery of the valuable minerals or satisfy product quality needs.”
There is a compromise between energy use and adequate liberation of the minerals to enable their recovery. Too little breakage results in minerals remaining trapped (unliberated) and not recovered or too coarse for the recovery process to select. Too much breakage results in loss through fines (slimes) that cannot be selected. The objective of breaking the rock into a narrow size range, well suited to recovery, is severely compromised by the relatively crude nature of the crushing, milling and classification equipment used for bulk processing of ores. Fine screens are providing greatly improved classification compared to hydro cyclones, but are currently only suited to lower throughput operations (less than 500 tph). However, they show greater promise in light of finding that the change in classification can improve the liberation characteristics of the ore, increasing recoveries while reducing energy needs (Frausto et al., 2021).
Powell has proposed flexible circuit design to improve the balance of energy applied to the rock, and enable early selection and progressive rejection of waste material. Considerable research and process development is being applied to coarser recovery processes, such as coarse flotation, that allow coarser product sizes to be recovered. Another approach to improving recoveries is by increasing the surface reactivity through mechanochemical treatment—the addition of chemical reagents combined with mechanical grinding (Dessemond et al., 2019).
Overall, the extraction of raw materials will remain essential to the production of batteries for many decades to come, as demand far outstrips available resource in the circular economy. Due to the high energy and environmental footprint of current production processes, improving the efficiency of this production is an important aspect of sustainable energy production.
2.2 Secondary raw materials from recycling
In order to achieve an economically and ecologically sustainable battery production, the recycling of the circulated lithium-ion batteries is of tremendous importance (Peters et al., 2017; Kwade and Diekmann, 2018; Zhao et al., 2020). According to Xu et al. (2020), the circulating batteries in consumer products, EV application and other use cases will increase many times by 2030. To cover the raw material demand in the targeted sustainable production of LIB, recycling represents a central and elementary bridge between LIB at end-of-life or production scrap, and the production of new cells (Habib et al., 2020).
2.2.1 Recycling impact on battery value chain
The key element to reduce the carbon footprint, minimize raw material dependencies and increase sustainability of batteries, is the recycling of the recyclables within the battery to reuse them in a new production cycle (Velázquez-Martínez et al., 2019).
Approaches to achieve this goal are described in the literature in wide variety, and are already successfully implemented at the research and, in some cases, the industrial level (Brückner et al., 2020; Velázquez-Martínez et al., 2019; Zeng et al., 2014; Arshad et al., 2020; Kwade and Diekmann, 2018). However, the ambitious targets of the EU or the CEID consortium (Kwade et al., 2020) with material recycling rates of up to 95 % of e.g. nickel (Ni), aluminum (Al), cobalt (Co), and 70 % of lithium, have not yet been achieved. Compared with other countries and regions of the world, the EU sets very high target recycling quotas with the proposed Battery Directive, which are also pursued equally by China. Producers are obliged to collect the batteries brought into circulation and return them to the material cycles. Other Asian countries aim to achieve similar goals (South Korean RoHS/ELV/WEEE Act, 2007 and Japan’s End-of-Life Vehicle Recycling Law).
With the help of the process steps for the LIB recycling, depending on the combination as depicted in Fig. 3, most of the valuable materials can be recovered and used for further battery applications or, if more meaningful, high-value application alternatives. With regard to the desired raw material-independent production sites of LIB, the process of recovery, after initial raw material supply to the producers, allows to both increase sustainability and environmental protection by decreasing the need for primary material from extraction sites, as well as to minimize the equipment required for production of the valuable metal salts for active material synthesis (Bonsu, 2020; Mathieux et al., 2017).
2.2.2 Potential LIB recycling processes
Today, LIBs with liquid electrolytes dominate the main market application (BEV, PHEV, portable devices and stationary energy storage). Regardless of the type of application, LIBs contain nickel, cobalt, manganese, aluminum, copper, graphite, polymeric and/or iron components, and in new technologies, silicon (Diekmann et al., 2017). From this point of view, it is clear that processes for recycling LIB’s are of enormous importance.
In principle, the processes of recycling LIB have a recurring scheme, which can be described independently from the chosen processing route. The first step is to deactivate the battery cells by discharging and short-circuiting them, by treating them with saline solution or pyrolyzing them at more than 200 °C (Georgi-Maschler et al., 2012; Hanisch et al., 2015a). Subsequently, four different process types can be used and combined in different ways: mechanical, thermal, pyrometallurgical and hydrometallurgical processes. Fig. 3 shows possible different process routes that can be applied. The individual processes allow only very limited possibilities to recover the materials, which is why a combination is essential. Pure pyrometallurgical processes (see Fig. 3(A)), as with direct smelting of LIB battery modules, are already industrially established, but allow only high recovery rates for transition metals such as nickel, cobalt and copper, whereas lithium in particular causes difficulties and is found especially within the resulting slag. To enable recovery of lithium and manganese along the way, the route has to be further linked to hydrometallurgical and undoubtedly mechanical processes as well. Other components such as graphite, organic solvents and polymers are thermally consumed as an energy source. For this reason, overall a relatively low total recovery rate can be assumed (Brückner et al., 2020).
Direct pyrometallurgical recycling can also be preceded by a mechanical crushing and sorting step, with subsequent pyrometallurgical treatment of the black mass or shredded battery material (see (C) and (D) in Fig. 3). The proportion of mechanical processing is a variable factor here. This pretreatment increases the recovery rate, as material such as polymers and aluminum can also be recovered.
Another route starts with the pyrolysis of the entire battery system to achieve safe handling in further process steps (see (B) in Fig. 3). By this route, all volatile components are removed, including a high portion of the fluorine containing components, which requires a complex gas cleaning. Afterwards, the parts of the battery system that are not burned are dismantled, and the cells are further mechanically processed using a shredder and separation devices, such as zig-zag-classifiers (more details see descriptions for route (B) in Chapter 2.2.3).
2.2.3 Mechanical-hydrometallurgical processing
A complex process route of LIB recycling is a combination of mechanical and hydrometallurgical steps, without an intermediate pyrometallurgical process, but with low temperature treatment or, eventually, a pyrolysis process step after shredding (Brückner et al., 2020; Georgi-Maschler et al., 2012; Kwade and Diekmann, 2018; Velázquez-Martínez et al., 2019). Upstream of mechanical processing, is a step to deactivate the modules and battery cells, which can be achieved by electrical deep discharge to 0 V and short circuiting, saline solutions, or shock freezing (Georgi-Maschler et al., 2012; Hanisch et al., 2015b; Shaw-Stewart et al., 2019). The discharged battery modules or cells are then crushed, the shredded product usually treated thermally, and the components are separated into the individual fractions in a sequence of different sorting and classification processes. The obtained black mass can be processed by hydrometallurgical processes to produce metal salts or graphite.
Two established, and largely mechanical processes for LIB recycling are the so-called LithoRec and Accurec processes. The LithoRec process was developed within the framework of public funding in Germany and subsequently commercialized within the company Duesenfeld GmbH, Wendeburg, Germany. The developed process chain, according Fig. 3(E) and Fig. 4, combines deep mechanical process steps with subsequent hydrometallurgy (Hanisch et al., 2015b; Diekmann et al., 2017; Kwade and Diekmann, 2018). In a first process step, the battery packs are electrically deep discharged and short-circuited. Material that has already been removed, such as the casing, battery management system and cables, can be forwarded for conventional recycling. The battery modules or cells are shredded in a 4-roller shredder or a rotor shear, whereby product sizes of 10 to 20 mm have been shown to be advantageous for the subsequent process steps according to Hanisch et al. (2015b); Kwade and Diekmann (2018). Due to the presence of electrolyte components (Ethylene carbonate (EC), Ethyl methyl carbonate (EMC), Dimethyl carbonate (DMC)), which pose in example a risk of flammability, it is recommended to carry out dry crushing in a nitrogen atmosphere (Kwade and Diekmann, 2018; Diekmann et al., 2020; Doose et al., 2021a). The comminution is followed by a drying step to remove electrolyte components. At temperatures of 100–140 °C, the highly volatile components DMC and EMC can be removed and recovered from the comminuted material (Stehmann et al., 2018). Alternatively, this can also be carried out in a vacuum drying process at reduced temperature, or at evaluated temperatures of more than 200 °C under inert or vacuum atmosphere (pyrolysis). The aim is to remove as much of the carbonates as possible, but to avoid the formation of reactive hydrogen fluoride (HF) from the conductive salt LiPF6 and PVDF binder. An optional mechanical mixing tool, used within the drying process, can also increase the detachment of active material from the conductor films, as well as the uniformity of thermal treatment of the material to be dried. This step is followed by mechanical separation of the present materials by way of a combination of air sifting by zig-zag-classifier, shredding and further sieving steps. In the first sifting step, components of the module and cell casings are removed from the shredded material. Subsequently, the remaining electrode foils, separator foil, polymeric components and black mass are shredded in a cutting mill to a maximum size of 10 mm. In addition, this process further promotes the detachment of black mass from the current collector foils. In a downstream air-sieving step, with sieve widths of 500 μm, the black mass is separated from the remaining fraction. The influence of additional 2nd comminution and separation of the components can be seen in Fig. 5. As the process depth increases, the percentage of fine material increases, which is mainly due to the increasing amount of black mass detached from the electrode foils. The black mass obtained can then be fed to hydrometallurgical processing for the recovery of Co, Ni, Mn and Li. In addition, the remaining fraction is separated from separator foil in a further sifting process and decoated aluminum and copper foil as well as plastic components can be forwarded for further commercial recycling (Diekmann et al., 2017; Kwade and Diekmann, 2018). The combination of these mechanical processes can result in more than a 90 % recovery of the black mass.


Another process already commercially established is the so-called Accurec recycling (Accurec GmbH, Krefeld, Germany) process for LIB, according to Fig. 3(B). The process combines thermal (pyrolysis, pyrometallurgical), mechanical and hydrometallurgical processes to recover valuable materials from LIBs. The process begins with the separation of battery cells and casing materials, as well as BMS and cables. These are subsequently fed into the conventional recycling processes. In the next process step, the dismantled battery cells are deactivated by means of a thermal vacuum step. In this batchwise process step, the cells are safely deactivated under vacuum with up to 600 C, in order to remove electrolyte components and volatile hydrocarbons (Brückner et al., 2020; Georgi-Maschler et al., 2012). The pyrolysis material obtained is subsequently fed into a milling process in order to separate the valuable materials from each other and make them accessible for mechanical separation processes. The processed material is separated in several separation steps with the help of vibrating sieves, magnetic separators and sifters. The exact process sequence and parameters are not described in detail in literature. With this process chain, the electrode active materials can be separated from iron, nickel, copper and aluminum components and processed separately in the following processes. The electrode active materials are subsequently pyrometallurgically processed at 800 °C under reducing conditions (e.g. regarding contained graphite from the anode) to form an alloy that may contain nickel, copper, cobalt and iron. The excess slag may contain lithium. Both fractions can subsequently be hydrometallurgically processed for high-value production, and transferred to a new application.
In addition to these two recycling process chains, there are further processes based essentially on mechanical processing that have already been developed, or are in development. For example, the Battery Resources “Closed Loop” process (Gratz et al., 2014), the OnTo Technology, and others that have already been established and whose function has been demonstrated.
2.2.4 Mechanical-pyrometallurgical processing
Commercially, the pyrometallurgical process route for recycling of LIBs has been established and implemented for the longest. In this process chain, the battery modules or cells can be processed directly by the pyrometallurgical high temperature treatment (see (A) in Fig. 3), or subjected to mechanical processing beforehand (see (C) and (D) in Fig. 3). With regard to an increased overall recycling rate, it has been shown to be advantageous to carry out mechanical processing beforehand (Brückner et al., 2020). Mechanical processing, from removal of the casing, to comminution of the modules and cells, and separation of the valuable materials into individual components, can be carried out analogously to the process steps used in mechanical-hydrometallurgical processing routes. The obtained black mass can be fed to pyrometallurgical processing. Of course, whole cells can also be given for further processing after removal of the casing. Pyrometallurgical processes include high temperature processes, such as melting and roasting of the materials to obtain battery slag, alloys and kiln dust. Black mass processing in pyrometallurgy begins with a heating phase in the range of 150–500 °C, during which electrolyte, binder and other remaining polymeric constituents are removed. In the next process phase, under reducing conditions, temperatures of up to 1450 °C are reached (by decomposing graphite in the black mass, coke, NH4Cl, CaCl2 and/or NaHSO4), and the products obtained are battery slag with Li-containing components LiO2 and Li2O3 and alloys. Ni, Co and Cu can be individually removed from the process and recovered, due to their different melting points. However, the other valuable materials (Li, Mn and Al) are contained in the kiln slag and/or the dust, and can only be recovered at high cost. The alloy, as well as the slag, and dust obtained are subsequently be processed hydrometallurgically, usually after milling.
As an industrial example, the process of Umicore ValéasTM (Bruxelles, Belgium) can be cited. The process is focused on the recovery of the metals Co and Ni from LIB and NiMH batteries, and is a combination of mechanical, pyro- and hydrometallurgical processes (Velázquez-Martínez et al., 2019). Compared to hydrometallurgical processing the pyrometallurgical processing of LIBs is very insensitive against different battery types, changing feed material and impurities. However, a subsequent hydrometallurgical treatment of the pyrometallurgical products obtained is necessary after pyrometallurgical processing, since intermediates that have no further industrial application are primarily produced (Brückner et al., 2020).
3. Active material production
To enable high growth in battery cell production, the availability of battery grade primary and secondary raw materials in form of metal salts, such as carbonates, sulphates and hydroxides (as basis for the cathode active materials), and graphites and silicon (as basis for the anode active materials) are essential.
3.1 Cathode active material
Common cathode active materials (CAM) are lithium metal oxides (e.g. LiNi1–x–yMnxCoyO2 and LiNi1–x–yCoxAlyO2, known as NMC and NCA, respectively) or lithium iron phosphates (e.g. LiFePO4, so-called LFP). The mean size, size distribution and morphology of CAM particles have a remarkable influence on the final structure, as well as on the resulting electrochemical performance of the lithium-ion battery (Ju and Kang, 2008; Sinha, 2009; Du et al., 2010). In fact, lower CAM particle sizes have shown to bring advantages, such as improved charge and discharge rates due to an increased electrolyte-CAM contact area. However, there are also some disadvantages, such as the increase of undesired side reactions due to a larger specific surface area. As a consequence, the ongoing formation and development of the solid electrolyte interface can occur, leading to self-discharge of the cell and thus, unwanted safety issues (Sinha, 2009). More specifically, targeted particle design has been addressed and directed through different techniques, the most common being milling and classification processes at various stages of CAM production.
The synthesis of CAM can be performed via different techniques. For NMC materials, for example, precipitation reactions forming hydroxides (Cao H. et al., 2005) or carbonates (Guo et al., 2011; Zhang Y. et al., 2006), sol-gel syntheses (Peng et al., 1998), hydrothermal syntheses (Burukhin, 2002), syntheses via spray drying, combustion reactions (Dahbi et al., 2012) and solid-state reactions (Gan et al., 2005) have already been investigated, achieving different product qualities. In addition, there are further methods for coating (Lu et al., 2013), doping (Naghash and Lee, 2001) and modification (Hashem et al., 2011) of the surfaces in order to enhance properties such as specific capacity, thermal stability or ionic conductivity. An overview of the process chain for the production of CAM, and the final application of milling and classification processes for precise control of particle size, is presented in Fig. 6.

Liu N. et al. (2014a) used a ball mill at the beginning of the synthesis route in order to pretreat a LiFePO4-precursor suspension (300 rpm with zirconia grinding balls of 1 mm diameter). This led to higher homogeneity and narrower size distribution in the subsequent spray drying synthesis process, which was combined with a carbon-thermal reduction to finally produce LiFePO4/C (carbon coating). They also demonstrated that smaller particles were correlated with a higher electrochemical performance. The initial discharge capacities of samples with particle sizes of about 12 μm reached about 158 mA h g−1, whereas those particles with sizes of 1.5 μm achieved 167.6 mA h g−1. They assumed that there was a decrease in the surface resistance, along with an improvement in the surface electronic conductivity, because of more homogeneous and complete carbon coating. Also, in this line of research work, Li H. et al. (2021) investigated the influence of ball milling after producing the precursor material by a co-precipitation process. After calcinating, the cathode material tailored by milling (Li1.2Ni0.13Co0.13Mn0.54O2) showed improved electrochemical properties.
Furthermore, ball milling is used to perform solid state synthesis by mixing and grinding all precursor materials at the same time (Cambaz et al., 2016; Kang et al., 2008; Yudha et al., 2019; Zhang Y. et al., 2006). A detailed understanding of the material manufacturing process can be found in the published studies of Kang et al. (2008) and Zhao et al. (2016), who varied the milling time in order to produce LiFePO4 and LiFePO4/C respectively. Kang et al. demonstrated that small crystallites are achieved through longer milling periods. Based on their results, they proposed that CAM particles with very low sizes may hinder the insertion and extraction of Li+ ions, and thus decrease the electrochemical performance. Nevertheless, by finding the optimum parameters of milling, along with optimized heat-treatment parameters, they were able to produce LiFePO4, which demonstrated a stable cycle performance and a discharge capacity of 160 mA g−1 at 0.1 C. Zhao et al. showed similar results, and additionally discovered that the effect is more significant at low temperatures (Zhao et al., 2016). This is caused by a hindrance of the lithium ion diffusivity, and an increment in the charge transfer resistance at the interfaces between electrode and electrolyte.
Furthermore, it has been demonstrated that calcinating the particles leads to an increase in their sizes, compared to the precursor material used, which results in a decreased specific capacity (Martha et al., 2009). Taking all these facts into consideration, controlling the final particle size distribution via a subsequent milling and classification processes is the key motivation of many studies (Ibarra-Palos et al., 2005; Kim et al., 2011; Stein IV et al., 2016). Milling parameters (e.g. process time, grinding media size and milling speed) have a direct impact on the size and morphology of the processed particles, a topic which has also been tackled by some researchers. In combination with comminution as a downstream process after synthesis, different classification processes are also used to achieve the target particle size. In particular, Stein IV et al. (2016) described the relationship between crystallite size and process parameters. Similar to the work of Kang et al. (2008), the smallest crystallite size did not lead to the best electrochemical performance, which proved to be significantly dependent on local structures within the electrode. These interdependencies between the grinding process and structural properties of active materials were thoroughly investigated by Pan et al. (2019). They varied the process times and identified relevant relationships with regard to the processing of NMC 442 cathode materials. Based on this study, it can be concluded that a reduction of the particle size can also be accompanied by structural changes in the form of a disordering of the layered structure, which then results in higher coulombic inefficiencies and capacity fading during cycling.
3.2 Anode active material
Graphite (natural and synthetic) is the most widely used active material on the anode side for lithium-ion batteries because of its good reversibility, stable voltage profile and good processing capabilities (Asenbauer et al., 2020). The high reversibility is due to the layered structure of graphite, in which the lithium ions can intercalate during charging and deintercalate during discharging of the battery (Yazami and Touzain, 1983). The intercalation process takes place step-wise, which is reflected as plateaus in the voltage profile. At the fully lithiated state, the theoretical specific capacity of graphite totals 372 mAh g−1 (Asenbauer et al., 2020). Despite its good reversibility and high specific capacity compared to the CAM, the anode is still in the focus of interest to increase the energy density (Wh/L) and performance of the cell. The two major research topics on the anode side are the increase of the specific capacity by using new active materials, like silicon or tin, and the reduction of the initial capacity loss from the formation of the Solid Electrolyte Interface (SEI). The SEI is formed in the first charge step as a consequence of the instability of the electrolyte in the voltage window of the anode, and consumes lithium, thus, reducing the amount of active lithium in the cell and the capacity (An et al., 2016; Ng et al., 2009). This initial loss of lithium is directly correlated to the specific surface area on the anode side, which should therefore be minimized (An et al., 2016; Wang H. et al., 1999).
In order to decrease the specific surface area, two different processes are applied: surface coating and mechanical spheronization of graphite particles. By coating the graphite particles with an additional layer, the nano- and micron-sized pores can be closed, and hence the specific surface area is decreased (Yoon et al., 2001). This process is only reasonable for blocking smaller pores, otherwise the coating thickness would become too large, with negative impacts on the ion transport and energy density (Asenbauer et al., 2020; Casino et al., 2020). In order to change the particle morphology, a mechanical spheronization can be used. An overview of the mechanical treatment of anode active materials is shown in Fig. 7.
3.3 Spheronization of graphite
Normally, graphite exhibits a flake-like structure, consisting of multiple stacked layers of graphene with a large thickness in the horizontal direction (basal plane) and low thickness in the vertical direction (Lämmerer and Flachberger, 2017). By spheronization using a series of impact milling and classification process steps, the graphite morphology is modified to a more spherical shape, often referred to as “potato”-like shape (Asenbauer et al., 2020; Lämmerer and Flachberger, 2017).
This resulting structure, with a reduced specific surface area, leads to various benefits compared to the pristine graphite material, e.g. higher tap-density (Asenbauer et al., 2020; Kwon et al., 2021; Lämmerer and Flachberger, 2017), lower electrode tortuosity (Ebner et al., 2014; Müller et al., 2018) and increased energy density (Müller et al., 2018). Furthermore, the winding of the graphene layers during spheronization leads to more superficially localized edge planes, enabling new active sites at the surface for lithium ion intercalation. This facilitates faster ion transport and, thus, a higher rate capability and specific capacity for the spheronized graphite particles (Lämmerer and Flachberger, 2017; Natarajan et al., 2001; Wu et al., 2011). In addition to the reduction of specific surface area, spheronization also diminishes and narrows the particle size distribution, both enabling a better cell performance and preventing lithium plating (Bläubaum et al., 2020).
However, with increasing stressing time within the impact mills, more and more basal planes are destroyed and the highly reactive edge planes created at the surface are prone to higher SEI growth and the exfoliation of graphite by the electrolyte and, therefore, diminishes the capacity again (Wang H. et al., 1999). Moreover, it was found that the open porosity in the structure (accessible for the electrolyte) increases, but the closed porosity (not accessible for the electrolyte) decreases with increasing stressing time (Mundszinger et al., 2017). Consequently, the process parameters and stressing times have to be carefully selected for optimized particle design and cell performance. Moreover, the spheronization is often combined with subsequent carbon-coating, to further decrease the specific surface area and contact of the superficial edge planes with the electrolyte (Lämmerer and Flachberger, 2017).
The preparation of these spherical graphite particles is often carried out via impact stressing of the graphite particles in fluidized bed mills, jet mills or classifier mills. In industrial applications, a cascade of several dry-operated classifier mills, as shown in Fig. 7 A), is mostly used. Within these classifier mills, a disc with grinding tools in a rectangular shape, or shaped as rounded blocks, rotates around a central classifier wheel. The grinding tools can be adjusted towards the breakage or deformation of the milling feed. For the purpose of spheronization, a rounded shape is preferred so that the particles are only plastically deformed, or the edges broken off. The particle fragments are removed by the integrated classifier, and the spheronized product remains in the process chamber. In the standard type of operation, the spheronization is carried out in a cascade of up to 20 mills. This process is, however, ineffective, because of its high operation cost, difficult scalability and low flexibility for change of product (Northern Graphite). The final yield amounts for only 30–50 %, meaning that more than half of the graphite used initially exits the process as fine powder, which cannot be used as active material anymore (Northern Graphite) (Steinrötter, 2011).
As previously discussed small particles exhibit a high specific surface area which enables on the one hand higher charging and discharging rates without lithium plating but on the other hand increases the irreversible capacity loss due to strong SEI growth (Bläubaum et al., 2020; Utsunomiya et al., 2011) and necessitates the usage of more binder leading to a decrease of energy density on electrode level due to larger inactive material content (Landesfeind et al., 2018). Furthermore, it was found that narrow particle size distributions in the single-digit micrometer range (x10 ≈ 4 μm, x50 ≈ 6 μm, x90 ≈ 9 μm) result in the best cell performance, among others highest specific capacity, cycling stability and C-rate capability (Bläubaum et al., 2020). Therefore, both mean particle size and width of particle size distribution are important factors for achieving a maximum possible cell performance.
Therefore, new classifier mills (e.g. FACULTY® S series from Hosokawa (HMC, 2021) and GyRhoy® from Netzsch) have been developed recently, which are able to replace these mill cascades and achieve the same throughput by reduced energy consumption and higher product yields. In contrast to the mill cascades, these mills operate in a batch or semi-batch mode.
Obrovac et al. (2020) developed a new simple method for spheronizing graphite particles by using ZrO2 as host particles in a dry micro-granulation. The graphite flakes roll up around the ZrO2 particles and form an additional layer with increasing thickness over time. At one point, this graphite layer becomes instable and flakes off as “potato”-shaped particles, which are then further rounded. Several other processes have been developed for the spheronization of graphite on laboratory scale, demonstrating superior characteristics of the structures obtained (Kwon et al., 2021; Lai et al., 2012; Wang H. et al., 1999).
3.4 Silicon: Next generation anode material
Silicon is a key material for many different application fields, because of its outstanding physical properties. It is the second most abundant element in the earth’s crust, and easily accessible. In the past ten years, the rising popularity of lithium-ion batteries (LIBs) led to growing interest in the use of silicon as an anode material, as the theoretical specific capacity of silicon (3580–4200 mAh/g) is roughly ten times higher than that of graphite (372 mAh/g) (Ashuri et al., 2016). During the charge/discharge cycles, silicon undergoes significant volume changes (up to 300 %). This leads to breakage of the silicon particles, resulting in continuous exposure of the active silicon surface and, thus, Lithium consumption through surface reactions. Moreover, the electrode structure becomes damaged, and results in loss of connection between particles and current collector, and destruction of the SEI. In the end, a silicon based battery shows poor cycling stability (Hu et al., 2014; Sun et al., 2016a).
In order to overcome the challenge of volume expansion and particle cracking, the failure mechanisms of silicon-based electrodes need to be understood in order to create a fully functioning silicon-based anode. For this purpose, the mechanisms during cycling of the battery must be investigated (Szczech and Jin, 2011). Sun et al. (2016a) discovered that pulverization of silicon particles and electrochemical passivation of some silicon particles lead to failure of the battery cell during lithiation. Liu and Huang (2011) investigated the charge and discharge behavior of batteries using silicon possessing varying sizes and structures. They could show that a critical particle diameter of 150 nm exists, at which no cracking during volume expansion occurs. This critical particle diameter is mentioned and confirmed in other publications (Liu X.H. et al., 2012; Sun et al., 2016b; Szczech and Jin, 2011). This also leads to the design of different nano structures, such as silicon based nanowires (Zhang C. et al., 2013a), nanotubes (Wu et al., 2012), nanosheets and nanoporous Si particles. Even though nano-sized silicon is able to maintain its structure without cracking, the problems from the large volume expansion (unstable formation of the SEI and contact loss of particles) still remains, although to a lesser extent. For that reason, silicon composite materials are targeted as promising solutions. Here, a composite material, often graphite, is used as framework for nano-sized silicon particles. The goal is to allow the silicon to expand inside the composite, while the electrode structure is maintained by the composite framework. By looking at the literature, the approach of customized composites is the most promising electrode design, both in terms of the electrochemical performance, as well as the mechanical integrity of silicon-based battery cells (see Chapter 3.5).
An important challenge is the production of nano-sized silicon, which can either be achieved via bottom-up or top-down processes. Bottom-up methods usually depend on chemical approaches, and often require long reaction times, expensive chemicals, complex process control and up-scaling difficulties (Hou et al., 2015; Nilssen et al., 2020). In top-down methods, coarser materials are broken down into smaller fragments by comminution. The choice of the right comminution machine highly depends on the size of the starting material.
Silicon is a rather hard and brittle material, rendering it suitable for grinding tasks. Leblanc et al. (2015) describe a comminution process route from silicon ingots, with sizes of a couple of centimeters down to the nanometer range. First, different silicon feedstocks, such as quartz or gravel (SiO2), were treated thermally in a furnace. By using a carbonaceous material, the silicon feedstock was converted to molten MG-silicon ingots. Subsequently, the ingots were crushed and ground in several dry grinding steps comprising of a jaw crusher, a roll mill and a jet mill. After these milling steps, the obtained silicon powder was in the range of a few micrometers (Leblanc et al., 2015). However, silicon in this size range is often already available from the semiconductor and photovoltaic industry, which cuts silicon wafers for their applications and produces pure micron-sized silicon dust as byproduct.
The subsequent nano-comminution is carried out in a solvent, in order to prevent agglomeration of the particles. Most commonly, stirred media mills are used for this task (Fig. 7 B)), and in the lab scale planetary ball mills may be employed. This type of mill was intensively investigated by researchers over the past decades (Breitung-Faes and Kwade, 2008; 2014; Knieke et al., 2010). Hou et al. (2015) applied a stirred media milling process for silicon, and investigated the structural changes and their impact on battery performance. They report that the bead milling strategy improved the battery performance because of amorphization of crystal structure, the formation of an oxidized surface (SiO2) and smaller particle size compared to micrometer-sized silicon. Early investigations of phase transition in silicon during bead milling were conducted by Gaffet and Harmelin (1990). A major challenge in nano-comminution is the stabilization of the ground particles. Here, the choice of an appropriate solvent is essential. Reindl et al. investigated the impact of dispersing silicon nanoparticles in different organic solvents, such as 1-butanol or toluene (Reindl et al., 2007; 2008). They found that the silicon particles have a pronounced tendency to agglomerate in organic solvents. They could stabilize the silicon nanoparticles by using fish oil as a steric stabilizing agent (Reindl et al., 2008). Nöske et al. (2019) applied a stirred media mill for the nano-comminution of silicon in ethanol. They performed an electrostatic stabilization, by preparing a sodium hydroxide solution in ethanol. The analysis of particle size and zeta-potential revealed that at pH*-values greater than 7.5 the silicon nanoparticles can be stabilized, and do not agglomerate. As a result, they suggest a two-step grinding process and electrostatic stabilization at pH* = 7.5. The results of their approach containing a pre- and fine grinding step can be seen in Fig. 8. Considering both grinding steps, the final product, particles with a size of x50,3 = 75 nm, is obtained after a specific energy input of 106 MJ kg−1 (Nöske et al., 2019).

The impact of electrostatic stabilization was also reported by Švrček et al. (2005). They claim that nano-sized silicon particles in ethanol could be stabilized by adding ammonia. Additionally, Nilssen and Kleiv (2020) investigated the impact of stabilization on the efficiency of silicon nano-comminution in stirred media mills. They could show that the lack of sufficient stabilization leads to an increase in the viscosity of the slurry. However, they also report that the non-stabilized slurry did not have a significant impact on the grinding efficiency. This was attributed to the pseudoplastic rheology of the slurry and high shear rates in the grinding chamber. Moreover, they investigated the importance of optimal stress energy on the grinding of silicon by varying the stirrer tip speed and size of the grinding media. Their parameter combinations suggest that at the lowest specific energy input, an optimum exists at a point of low stress energy (Nilssen et al., 2020; Nilssen and Kleiv, 2020). From a production point of view, this means a longer overall grinding or production time, but less product contamination caused by grinding media wear. The impact of wear from silicon grinding on battery performance is still being investigated. In general, the choice of appropriate grinding parameters is a compromise between productivity and product purity (Nilssen et al., 2020).
3.5 Silicon composites
Even though silicon is the most promising anode material for the future, silicon has only been applied in small amounts (typically <5 wt %) in commercial cells (Choi and Aurbach, 2016). As previously discussed, this is mainly due to the challenges arising from the large volume change of silicon during lithiation (up to 300 %), whereas graphite shows only minor volume expansion of 10 %. Therefore, the combination of silicon and graphite has attracted much attention. While graphite with its high electrical conductivity, good cycling characteristics and low volume expansion provides a stable matrix, even small amounts of silicon can significantly enhance the capacity. This can be illustrated by the fact that by using 10 wt % silicon, the specific capacity of the anode can be nearly doubled.
In current commercial cell manufacturing, small silicon contents are simply blended with the graphite active material during electrode production (Choi and Aurbach, 2016). However, when using larger silicon contents, the resulting cell performance is poor and requires an optimized particle design. Therefore, multiple composite structures for silicon and silicon graphite composites have been developed, and been proven to be beneficial for capacity retention. These structures can be divided into three main approaches (Franco Gonzalez et al., 2017; He et al., 2021; Jin et al., 2017): nanostructures, porous and other 3D-structures and Si/SiOx composites.
Two different composite structures comprising silicon nanoparticles are possible: a core-shell structure with a layer of silicon nanoparticles on graphite, or the encapsulation of the nanoparticles (Li M. et al., 2014; Müller et al., 2021; Sui et al., 2018). Müller et al. demonstrated a good performance, for which silicon nanoparticles are prepared beforehand by grinding in a stirred media mill, and then coated onto coarser graphite particles by scalable fluidized bed granulation (Si@Gr). After subsequent pitch-coating (Si@Gr/C), the composite demonstrated good cycling stability and rate performance (Müller et al., 2021). The manufacturing process of these composites is shown in Fig. 7 C).
Additionally, silicon nanoparticles can be encapsulated in hollow carbon spheres. The silicon particles can expand in the highly conductive carbon matrix without any volume change of the composite particle itself. This characteristic is advantageous, because it prevents electrical isolation even after pulverization and allows for a stable SEI layer and superior cycling characteristics (Liu Y. et al., 2014b).
4. Electrode slurry production
The production of electrodes initially requires the classical mechanical process of dispersive mixing to obtain a coatable slurry (Kwade et al., 2018; Liu H. et al., 2021; Wenzel et al., 2015). In this context dispersive mixing means that beside a homogenization of the material mixture (i.e. a distributive mixing) material components are reduced in their size and changed in their structure by mechanical stresses and, thus, comminution of these material components. Here the fine carbon black (CB) agglomerates and aggregates have to be reduced in size by breaking off solid bridges and van der Waals based adhesion between the CB primary particles. Typically, after a dry homogenization, and an optional dry intensive, i.e. dispersive mixing, the powder components (active materials (AM), conductive agents like carbon black (CB) and polymeric binders) are mixed with a solvent and homogeneously distributed in it. During the dispersive mixing, the main objective is to fragment the carbon black agglomerates, and to comminute CB aggregates to defined sizes and structures, without damaging the other components (Bockholt et al., 2016b; Wenzel et al., 2015). Particularly in combination with polymer binders, the size and distribution of CB defines the formation of the microstructural network in the slurry, and later the electrode (Gaikwad and Arias, 2017). This microstructure determines the resulting electrode properties and the cell performance (Bockholt et al., 2016b; Zhang C. et al., 2013a), which turns the process steps of dispersive mixing into a central and property-defining part of the knowledge-based production of high quality LIB electrodes. The type of conductive additives and binder systems used will vary depending on the electrode type (Zhang Q. et al., 2013b). For example, polyvinylidene fluoride (PVDF) binders in combination with different types of CB and conductive graphite (CG) are used in cathode slurries. According to recent literature, cathode slurries are mostly N-2-methylpyrrolidone-(NMP)-based, while anode slurries are processed with water, using a binder system of carboxymethyl cellulose (CMC) and styrene butadiene rubber (SBR) (Gordon et al., 2020; Kwade et al., 2018).
4.1 State of technology
Due to the highlighted significance of size and distribution of conductive additives for the microstructural formation, the characterization of conductive additives before, during, and after mixing is of particular importance. Thus, a methodology via light diffraction and scattering for determining the size of CB particles from electrode slurries, including a sample preparation routine, was developed and tested (Dreger et al., 2017). In addition, other studies identify the condition and effects of CB via electrical, viscometric, imaging, or ultrasound scattering techniques. During dispersion, the formulation strategy and the process parameters determine the particle size and distribution (Wenzel et al., 2014). Thus, numerous publications focus on the relationship of processing and electrode slurry properties. For example, the importance, and subsequent consequences, of formulation strategies were shown. Here, optimal electrochemical rate performance was achieved due to ideal electrode porosities by utilizing moderate dispersing intensities for dry and binder type formulations (Haselrieder et al., 2014). In order to set the CB size, an additional dry mixing step prior to dispersion in a (high) intensity mixer can be applied: prolonged exposure of the components leads to a lower density of the coating due to increased deagglomeration of CB, and ultimately to increased adhesive strengths and improved discharge capacities and cycle stabilities (Bockholt et al., 2016a). These effects generally result in lower porosities and higher cohesiveness of the electrode coating. Additionally, CB attaches to the AM particle surfaces, establishing short-range electrical contacts all around the NMC particles (Bauer et al., 2015; Bockholt et al., 2016a). These CB-coated AM particles can also be achieved by dispersion processes without prior dry mixing. For example, kneading processes in planetary mixers are suitable for high-intensity stressing of slurries at high solids contents (~80 wt % and above), favoring very small sizes of CB in the range of 100 nm, which are particularly important for the formation of short-range conductive paths (Mayer et al., 2020). Process control in batch mixers concerning the dispersing intensity is also proving to be very important for optimizing electrodes, specifically with thicker layers for high-energy applications (Kremer et al., 2020).
For the dispersive mixing, the specific energy is a key evaluation value. It can be determined by the power input of the machine, minus the no-load power consumption, summed over time, in relation to the mass of the slurry. To highlight the role of the specific energy input, Fig. 9 shows exemplary results for CB deagglomeration with a dissolver (Dispermat AE03, VMA Getzmann) and a planetary mixer (PMH10, NETZSCH) by relating the decreasing volumetric median of the typically bimodal particle size distribution of CB, determined as described by Dreger et al. (2017), to the specific energy. The influence of the dispersing machines is directly evident: In the planetary mixer, the mixing tool velocities affect the final particle sizes of CB that can be achieved. In the dissolver, however, the particle size is exclusively dependent on the specific energy. Additionally, the dissolver shows a higher energy efficiency, but is less suitable for scaling to industrial standards due to its poorer distributive mixing properties.

Based on such experimental results, model-like descriptions of the dispersion of LIB slurries can be created purely empirically (Grießl et al., 2021), or by means of classical relationships of particle stressing in slurries (approaches according to Kolmogorov (1958); Krekel (1966); Kwade (2003)). Dispersion models enable knowledge-based scaling of the process machines, and converting discontinuous into continuous processes, which will become increasingly important in the future.
4.2 Future perspective
In order to increase the economic efficiency of the dispersion process and, thus, enable large-scale production, even at labor expensive locations, the conversion of typical batch mixers used industrially into continuous mixing processes is advised (e.g. using twin-screw extruders (Schunemann et al., 2016)). Accordingly, the application of extruders for electrode production is increasingly moving into the focus of research and process development. For instance, a laboratory-scale dissolver process was compared to a discontinuous kneading and a continuous extrusion process. The continuous production of slurries by the low-solvent extrusion processes exhibit good electrochemical performance, showing good deagglomeration and almost complete coverage of active material particles by CB (Dreger et al., 2015). Further investigations showed the decisive influence of the solids content on the extrusion-based production of cathode slurries. Higher solids contents reduce sedimentation effects and binder migration. In addition, they define the fragmentation of the conductive CB and, thus, the resulting properties of manufactured cathodes (Haarmann et al., 2020). Other works show the use, or combination, of batch and continuous processes. The powder components are prepared for a subsequent extrusion-based coating process by means of intensive mixers and kneaders, whereby the actual dispersion takes place before the extrusion process (Seeba et al., 2020). Beside extrusion, the dispersing in continuously operated stirred media mills was also reported from industry.
On the material side, due to their advantageous properties, fiber-like conductive additives, such as carbon nano tubes (CNT) and vapor-grown carbon fibers (VGCF), are increasingly used in research and industrial applications as a supplement, or a complete replacement, for conductive CB (Landi et al., 2009; Park et al., 2018; Qi et al., 2015). CNTs are only needed in small quantities, but are in some cases more expensive than CB (Bauer et al., 2015). LIB cathodes with CNTs show low initial Coulomb efficiencies and large voltage hysteresis, but exceedingly high electrical conductivity (Bauer et al., 2015). This advantage results from the ability to connect several active material particles within the electrode structure due to its length and also obtain an improved cycle stability and C-rate performance (Huang et al., 2010; Zhang Q. et al., 2013b). For the dispersing of the fiber-like conductive additives, high-pressure homogenizers are a well-suited alternative to conventional equipment. In terms of cathode active materials, adaptations will be necessary in the future for the more nickel-rich layered oxides (e.g. NCM811), which are both sensitive to moisture and mechanically more unstable (Heck et al., 2020).
5. Outlook—Solid electrolytes
5.1 Mechanochemical synthesis
In order to enable scalable production of solid-state batteries, solid electrolytes are required in sufficient quantities and with high and consistent quality. Although planetary ball mills, vibrating ball mills, and stirred media mills should all be suitable for mechanochemical syntheses of solid electrolytes, so far planetary ball mills have almost always been used on a laboratory scale (Schlem et al., 2021b).
The reasons might be the simple experimental setup, as well as the wide-spread use. As their ability for large-scale synthesis is quite poor, a transfer to continuous ball/bead mill types (e.g. stirred media mills) seems desirable. Most publications on mechanochemical routes consider the effect of processing parameters and conditions for the conversion time, instead of energy input. As this makes it difficult to distinguish between effects of energy dissipation and activation thresholds, and simply faster mechanical processing, further investigations with focus on process technology are still required.
Among solid electrolytes, thiophosphates and sulfides represent the material system considered the most promising class of materials, with very high Li ionic conductivities up to 10−2 S/cm and beyond (Kato et al., 2016). They are usually produced by classical solid-state syntheses via dry process routes (Dietrich et al., 2017; Kraft et al., 2018), or via solvent-based processes (Ghidiu et al., 2019). To ensure sufficient ionic conductivity, σion, and electrochemical stability (e.g. against high-voltage cathode materials), the absence of possible impurities is especially crucial. The latter can result from solvents used in the synthesis process and necessitate thorough washing (Kudu et al., 2018). While classical solid-state synthesis in sealed quartz ampoules does not seem suitable for scalable production (Kudu et al., 2018), mechanochemical synthesis within mills is a promising approach. Compared to the alternative methods, the products have predominantly amorphous structures, due to the kinetically controlled reactions, but can often be crystallized by a subsequent annealing step (Gautam et al., 2019). Sulfide electrolytes can be prepared as pure Li-P-S phases from starting materials Li2S and P2S5 with different stoichiometries (Dietrich et al., 2017; Tatsumisago et al., 2002), or in combination with metals (e.g. Si or Ge), or halides (Cl, Br, I) (Zhang Z. et al., 2018). Compared to classical solid-state syntheses, mechanochemical methods allow the synthesis of additional metastable compounds and local structures (Stöffler et al., 2019). Of particular importance are the argyrodites, Li6PS5Cl and Li6PS5Br, as well as their variants with iso- and aliovalent substitutions, in whose synthesis an annealing step follows a ball-milling process (Kraft et al., 2018; Rao and Adams, 2011). A significantly poorer σion is exhibited by Li6PS5I (Hanghofer et al., 2019), although σion here can be increased by two orders of magnitude by subsequent “soft” ball-milling of 120 min at 400 rpm (Brinek et al., 2020). This is attributed to the transition from micro- to nanocrystallinity. However, it is by no means the case that mechanochemical stress leads to an improvement of the material in every case: as shown by Dewald et al. (2021), ball-milling of Li6PS5Cl synthesized via classical solid-state reaction leads to a decrease in σion.
Among solid electrolytes for sodium batteries, Na3PS4 is frequently synthesized mechanochemically (Nguyen et al., 2019; Takeuchi et al., 2018). Nguyen et al. (2019) thoroughly studied the influence of milling media size, disc rotation speed, and process time in a planetary ball mill. They found that the same σion can be obtained by different synthesis conditions. The mechanochemically prepared compounds mostly have higher σion, which is attributed to different achievable crystal structures, as well as disorder in the structure of Na+ (Krauskopf et al., 2018). Mechanochemical methods are also well suited for introducing defects by doping, achieving σion as high as 41 mS/cm (Fuchs et al., 2020; Hayashi et al., 2019). The relationship between the structure influenced by the mechanochemical processes—micro-, macrostrain, and defects—and ion transport is studied in detail by Famprikis et al. (2020).
Another class of solid electrolytes that can be produced via mechanochemical reactions are halides (Asano et al., 2018; Li X. et al., 2019). They provide high electrochemical stability against cathode active materials, as well as mechanical deformability at room temperature (Li X. et al., 2020). Schlem et al. (2020) showed that for Li3ErCl6 and Li3YCl6, cation disorder induced by ball-milling leads to an increase in σion. In contrast, for Li3YBr6 the σion can be increased by subsequent annealing (Schlem et al., 2021a). Recently, it was demonstrated that a halide superionic conductor without rare earth metals, namely Li2.25Zr0.75Fe0.25Cl6, with a Li+ conductivity up to ≈1 mS cm−1, could be prepared in a planetary ball mill (Kwak et al., 2021).
In order to combine the advantages of different types of solid electrolytes (i.e. the high σion at room temperature of inorganic electrolytes and the good processability of polymer electrolytes) so-called hybrid electrolytes are produced. For example, the reaction of 70Li2S·30P2S5 (mol%) and different –OH group terminated oligomers in a planetary ball mill can lead to the formation of P–O–C bonds. To combine an oxide electrolyte with polymer, LLZO was mixed with an acetonitrile solution of PEO and LiClO4, and subsequently uniformly homogenized in a low energy ball mill (Dixit et al., 2019). The slurry obtained was subsequently coated via coextrusion, demonstrating a scalable manufacturing process.
5.2 Conditioning
For use in composite cathodes or separators, in addition to the (electro)chemical properties, the particle size of the solid electrolyte is crucial (Kerman et al., 2017). For example, a particle size smaller than 1 μm allows a more uniform distribution in the cathode and, thus, a better contacting of the active material (Calpa et al., 2019). Dixit et al. (2020) conditioned the microstructure and interface between the separator and the metallic lithium anode using iodine doping, as well as ball-milling and annealing. They milled an LPS/0.5LiI mixture together with heptane for 12 h, at a rather low rotation speed of 200 rpm, to reduce the particle size.
In addition, mechanochemical methods can be used to obtain desirable cathode composite microstructures, which are crucial for the performance of all solid-state batteries (Nagao et al., 2012). A ball-milled cathode composite for Li-S solid-state batteries consisting of Li6PS5Cl and sulfur-carbon composite outperformed a hand-ground one with respect to initial capacity, as well as capacity retention (Ohno et al., 2019). This is attributed to the grinding of the active material, leading to intimate contact in the composite, which helps to mitigate volume changes during cycling. An alternative to the application of ball mills is dry-coating of the active material particles with the solid electrolyte by a dry blending device (Nara Machinery Co., Ltd.) to achieve a high contact area, as well as well-percolated ion transport pathways (Nakamura et al., 2020).
Graebe et al. (2017) reported the use of a centrifugal mixer for the preparation of a composite material for a solvent-free manufacture of polyethylene oxide (PEO) based solid state electrodes. This dry mixing and mechanofusion process step is the key challenging step, as it ensures a free-flowing powder, necessary to produce a dense solid-state cathode or solid-state separator. This is accomplished by coating the PEO secondary particles with SiO2 or C65 to prevent further agglomeration.
6. Conclusion and outlook
The increasing demand for lithium-ion-batteries and future types of lithium batteries, especially for the ramp-up of electromobility worldwide, requires the further development of an economically and, at the same time, ecologically sustainable supply and production chain, starting with the extraction and processing of the primary and secondary raw materials, over their refinement to battery grade intermediates, to active and passive materials in the production of the cell components, like electrodes and finally battery cells. However, sustainability can only be reached if the end-of-life battery systems are used as secondary material source for the production of new battery cells, through which the recycling of the materials and the re-synthesis or re-conditioning of the active materials, especially for the cathode, becomes mandatory. Accordingly, regulations will be issued by the governments, like the EU, to ensure very high recovery rates for the entire battery system and especially for the valuable metals. Latest by this demand milling, dispersing and classification processes get a central role within the circular processing of lithium-ion batteries and next battery generations.
However, as shown in the review, comminution and classification is not only very central for the primary and secondary raw material processing, but also for the production of the cathode and anode active materials and, in the future, the mechanochemical synthesis of the solid electrolytes. The dry and wet mixing and dispersion of the electrode material mixture requires processes in which the particles are stressed, so that besides different types of mixers, extruders and wet operated mills are also used for the wet dispersion of components, especially in the case of nano-sized components. The mill and classifier manufacturers, the plant designers and builders, as well as the research institutions, should embrace these opportunities and develop new machinery and new processes to significantly increase the sustainability of circular battery cell production. In the near future, great attention should lie on energy efficient recycling processes, which ensure a high recycling rate of nearly 100 % and high component purity, as well as processes for dry electrode processing, where the dry pretreatment of the material mixture is a crucial process step. Moreover, the tailoring of the particle size distributions and particle morphologies, especially for new material classes like monocrystalline cathode active material and solid electrolytes, is an important task for the particle technology community.
Acknowledgements
Many thanks to Michael Grube, Moritz Hofer and Clara Sangrós for their input to this review in the field of active materials and the production of sulphidic solid electrolytes as well as Heather Cavers for an overall review of the spelling.
References
- Amarante M.M., Sousa A.B. de, Leite M.M., Processing a spodumene ore to obtain lithium concentrates for addition to glass and ceramic bodies, Minerals Engineering, 12 (1999) 433–436. DOI:10.1016/S0892-6875(99)00023-0
- Amer A.M., The hydrometallurgical extraction of lithium from Egyptian montmorillonite-type clay, JOM, 60 (2008) 55–57. DOI:10.1007/s11837-008-0137-5
- An S.J., Li J., Daniel C., Mohanty D., Nagpure S., Wood D.L., The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling, Carbon, 105 (2016) 52–76. DOI:10.1016/j.carbon.2016.04.008
- Arshad F., Li L., Amin K., Fan E., Manurkar N., Ahmad A., Yang J., Wu F., Chen R., A comprehensive review of the advancement in recycling the anode and electrolyte from spent lithium ion batteries, ACS Sustainable Chemistry & Engineering, 8 (2020) 13527–13554. DOI:10.1021/acssuschemeng.0c04940
- Asano T., Sakai A., Ouchi S., Sakaida M., Miyazaki A., Hasegawa S., Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries, Advanced Materials, 30 (2018) 1803075. DOI:10.1002/adma.201803075
- Asenbauer J., Eisenmann T., Kuenzel M., Kazzazi A., Chen Z., Bresser D., The success story of graphite as a lithium-ion anode material – fundamentals, remaining challenges, and recent developments including silicon (oxide) composites, Sustainable Energy & Fuels, 4 (2020) 5387–5416. DOI:10.1039/d0se00175a
- Ashuri M., He Q., Shaw L.L., Silicon as a potential anode material for Li-ion batteries: where size, geometry and structure matter, Nanoscale, 8 (2016) 74–103. DOI:10.1039/c5nr05116a
- Baláž P., Mechanical activation in hydrometallurgy, International Journal of Mineral Processing, 72 (2003) 341–354. DOI:10.1016/S0301-7516(03)00109-1
- Ballantyne G.R., Peukert W., Powell M.S., Size specific energy (SSE)—energy required to generate minus 75 micron material, International Journal of Mineral Processing, 136 (2015) 2–6. DOI:10.1016/j.minpro.2014.09.010
- Ballantyne G.R., Powell M.S., Benchmarking comminution energy consumption for the processing of copper and gold ores, Minerals Engineering, 65 (2014) 109–114. DOI:10.1016/j.mineng.2014.05.017
- Barbosa L.I., Valente G., Orosco R.P., González J.A., Lithium extraction from β-spodumene through chlorination with chlorine gas, Minerals Engineering, 56 (2014) 29–34. DOI:10.1016/j.mineng.2013.10.026
- Bauer W., Nötzel D., Wenzel V., Nirschl H., Influence of dry mixing and distribution of conductive additives in cathodes for lithium ion batteries, Journal of Power Sources, 288 (2015) 359–367. DOI:10.1016/j.jpowsour.2015.04.081
- Bläubaum L., Röder F., Nowak C., Chan H.S., Kwade A., Krewer U., Impact of particle size distribution on performance of lithium-ion batteries, ChemElectroChem, 7 (2020) 4755–4766. DOI:10.1002/celc.202001249
- Bloomberg New Energy Finance, Electric Vehicle Outlook 2018 (summary), (2018). Retrieved from mm<https://bnef.turtl.co/story/evo2018?teaser=true> accessed17032022.
- Bockholt H., Haselrieder W., Kwade A., Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes, Powder Technology, 297 (2016a)266–274. DOI:10.1016/j.powtec.2016.04.011
- Bockholt H., Indrikova M., Netz A., Golks F., Kwade A., The interaction of consecutive process steps in the manufacturing of lithium-ion battery electrodes with regard to structural and electrochemical properties, Journal of Power Sources, 325 (2016b)140–151. DOI:10.1016/j.jpowsour.2016.05.127
- Bonsu N.O., Towards a circular and low-carbon economy: Insights from the transitioning to electric vehicles and net zero economy, Journal of Cleaner Production, 256 (2020) 120659. DOI:10.1016/j.jclepro.2020.120659
- Breitung-Faes S., Kwade A., Nano particle production in high-power-density mills, Chemical Engineering Research and Design, 86 (2008) 390–394. DOI:10.1016/j.cherd.2007.11.006
- Breitung-Faes S., Kwade A., Use of an enhanced stress model for the optimization of wet stirred media milling processes, Chemical Engineering & Technology, 37 (2014) 819–826. DOI:10.1002/ceat.201300686
- Brinek M., Hiebl C., Wilkening H.M.R., Understanding the origin of enhanced Li-ion transport in nanocrystalline argyrodite-type Li6PS5I, Chemistry of Materials, 32 (2020) 4754–4766. DOI:10.1021/acs.chemmater.0c01367
- Brückner L., Frank J., Elwert T., Industrial recycling of lithium-ion batteries—a critical review of metallurgical process routes, Metals, 10 (2020) 1107. DOI:10.3390/met10081107
- Burukhin A., Hydrothermal synthesis of LiCoO2 for lithium rechargeable batteries, Solid State Ionics, 151 (2002) 259–263. DOI:10.1016/S0167-2738(02)00721-X
- Calpa M., Rosero-Navarro N.C., Miura A., Tadanaga K., Electrochemical performance of bulk-type all-solid-state batteries using small-sized Li7P3S11 solid electrolyte prepared by liquid phase as the ionic conductor in the composite cathode, Electrochimica Acta, 296 (2019) 473–480. DOI:10.1016/j.electacta.2018.11.035
- Cambaz M.A., Anji Reddy M., Vinayan B.P., Witte R., Pohl A., Mu X., Chakravadhanula V.S.K., Kübel C., Fichtner M., Mechanical milling assisted synthesis and electrochemical performance of high capacity lifebo3 for lithium batteries, ACS applied materials & interfaces, 8 (2016) 2166–2172. DOI:10.1021/acsami.5b10747
- Cao H., Zhang Y., Zhang J., Xia B., Synthesis and electrochemical characteristics of layered LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries, Solid State Ionics, 176 (2005) 1207–1211. DOI:10.1016/j.ssi.2005.02.023
- Casino S., Niehoff P., Börner M., Winter M., Protective coatings on silicon particles and their effect on energy density and specific energy in lithium ion battery cells – a model study, Journal of Energy Storage, 29 (2020) 101376. DOI:10.1016/j.est.2020.101376
- Choi J.W., Aurbach D., Promise and reality of post-lithium-ion batteries with high energy densities, Nature Reviews Materials, 1 (2016) 16013. DOI:10.1038/natrevmats.2016.13
- Dahbi M., Wikberg J.M., Saadoune I., Gustafsson T., Svedlindh P., Edström K., Electrochemical behavior of LiNi1–y–zCoyMnzO2 probed through structural and magnetic properties, Journal of Applied Physics, 111 (2012) 23904. DOI:10.1063/1.3676434
- Dessemond C., Lajoie-Leroux F., Soucy G., Laroche N., Magnan J.-F., Spodumene: the lithium market, resources and processes, Minerals, 9 (2019) 334. DOI:10.3390/min9060334
- Dewald G.F., Ohno S., Hering J.G.C., Janek J., Zeier W.G., Analysis of charge carrier transport toward optimized cathode composites for all-solid-state Li–S batteries, Batteries & Supercaps, 4 (2021) 183–194. DOI:10.1002/batt.202000194
- Diekmann J., Doose S., Weber S., Münch S., Haselrieder W., Kwade A., Development of a new procedure for nail penetration of lithium-ion cells to obtain meaningful and reproducible results, Journal of The Electrochemical Society, 167 (2020) 90504. DOI:10.1149/1945-7111/ab78ff
- Diekmann J., Hanisch C., Froböse L., Schälicke G., Loellhoeffel T., Fölster A.-S., Kwade A., Ecological recycling of lithium-ion batteries from electric vehicles with focus on mechanical processes, Journal of The Electrochemical Society, 164 (2017) A6184–A6191. DOI:10.1149/2.0271701jes
- Dietrich C., Weber D.A., Sedlmaier S.J., Indris S., Culver S.P., Walter D., Janek J., Zeier W.G., Lithium ion conductivity in Li2S–P2S5 glasses – building units and local structure evolution during the crystallization of superionic conductors Li3PS4, Li7P3S11 and Li4P2S7, Journal of Materials Chemistry A, 5 (2017) 18111–18119. DOI:10.1039/C7TA06067J
- Dixit M.B., Singh N., Horwath J.P., Shevchenko P.D., Jones M., Stach E.A., Arthur T.S., Hatzell K.B., In situ investigation of chemomechanical effects in thiophosphate solid electrolytes, Matter, 3 (2020) 2138–2159. DOI:10.1016/j.matt.2020.09.018
- Dixit M.B., Zaman W., Bootwala Y., Zheng Y., Hatzell M.C., Hatzell K.B., Scalable manufacturing of hybrid solid electrolytes with interface control, ACS Applied Materials & Interfaces, 11 (2019) 45087–45097. DOI:10.1021/acsami.9b15463
- Doose S., Haselrieder W., Kwade A., Effects of the nail geometry and humidity on the nail penetration of high-energy density lithium ion batteries, Batteries, 7 (2021a) 6. DOI:10.3390/batteries7010006
- Doose S., Mayer J.K., Michalowski P., Kwade A., Challenges in ecofriendly battery recycling and closed material cycles: a perspective on future lithium battery generations, Metals, 11 (2021b) 291. DOI:10.3390/met11020291
- Dreger H., Bockholt H., Haselrieder W., Kwade A., Discontinuous and continuous processing of low-solvent battery slurries for lithium nickel cobalt manganese oxide electrodes, Journal of Electronic Materials, 44 (2015) 4434–4443. DOI:10.1007/s11664-015-3981-4
- Dreger H., Huelsebrock M., Froboese L., Kwade A., Method development for quality control of suspensions for lithium-ion battery electrodes, Industrial & Engineering Chemistry Research, 56 (2017) 2466–2474. DOI:10.1021/acs.iecr.6b02103
- Du W., Gupta A., Zhang X., Sastry A.M., Shyy W., Effect of cycling rate, particle size and transport properties on lithium-ion cathode performance, International Journal of Heat and Mass Transfer, 53 (2010) 3552–3561. DOI:10.1016/j.ijheatmasstransfer.2010.04.017
- Ebner M., Chung D.-W., García R.E., Wood V., Tortuosity anisotropy in lithium-ion battery electrodes, Advanced Energy Materials, 4 (2014) 1301278. DOI:10.1002/aenm.201301278
- Famprikis T., Kudu Ö.U., Dawson J.A., Canepa P., Fauth F., Suard E., Zbiri M., Dambournet D., Borkiewicz O.J., Bouyanfif H., Emge S.P., Cretu S., Chotard J.-N., Grey C.P., Zeier W.G., Islam M.S., Masquelier C., Under pressure: mechanochemical effects on structure and ion conduction in the sodium-ion solid electrolyte Na3PS4, Journal of the American Chemical Society, 142 (2020) 18422–18436. DOI:10.1021/jacs.0c06668
- Franco Gonzalez A., Yang N.-H., Liu R.-S., Silicon anode design for lithium-ion batteries: progress and perspectives, The Journal of Physical Chemistry C, 121 (2017) 27775–27787. DOI:10.1021/acs.jpcc.7b07793
- Frausto J.J., Ballantyne G.R., Runge K., Powell M.S., Wightman E.M., Evans C.L., Gonzalez P., Gomez S., The effect of screen versus cyclone classification on the mineral liberation properties of a polymetallic ore, Minerals Engineering, 169 (2021) 106930. DOI:10.1016/j.mineng.2021.106930
- Fuchs T., Culver S.P., Till P., Zeier W.G., Defect-mediated conductivity enhancements in Na3–xPn1–xWxS4 (Pn = P, Sb) using aliovalent substitutions, ACS Energy Letters, 5 (2020) 146–151. DOI:10.1021/acsenergylett.9b02537
- Gaffet E., Harmelin M., Crystal-amorphous phase transition induced by ball-milling in silicon, Journal of the Less Common Metals, 157 (1990) 201–222. DOI:10.1016/0022-5088(90)90176-K
- Gaikwad A.M., Arias A.C., Understanding the effects of electrode formulation on the mechanical strength of composite electrodes for flexible batteries, ACS applied materials & interfaces, 9 (2017) 6390–6400. DOI:10.1021/acsami.6b14719
- Gan C., Hu X., Zhan H., Zhou Y., Synthesis and characterization of Li1.2Ni0.6Co0.2Mn0.2O2+δ as a cathode material for secondary lithium batteries, Solid State Ionics, 176 (2005) 687–692. DOI:10.1016/j.ssi.2004.10.021
- Gautam A., Sadowski M., Prinz N., Eickhoff H., Minafra N., Ghidiu M., Culver S.P., Albe K., Fässler T.F., Zobel M., Zeier W.G., Rapid crystallization and kinetic freezing of site-disorder in the lithium superionic argyrodite Li6PS5Br, Chemistry of Materials, 31 (2019) 10178–10185. DOI:10.1021/acs.chemmater.9b03852
- Georgi-Maschler T., Friedrich B., Weyhe R., Heegn H., Rutz M., Development of a recycling process for Li-ion batteries, Journal of Power Sources, 207 (2012) 173–182. DOI:10.1016/j.jpowsour.2012.01.152
- Ghidiu M., Ruhl J., Culver S. P., Zeier W. G., Solution-based synthesis of lithium thiophosphate superionic conductors for solid-state batteries: a chemistry perspective, Journal of Materials Chemistry A, 7 (2019) 17735–17753. DOI:10.1039/C9TA04772G
- Gordon R., Orias R., Willenbacher N., Effect of carboxymethyl cellulose on the flow behavior of lithium-ion battery anode slurries and the electrical as well as mechanical properties of corresponding dry layers, Journal of Materials Science, 55 (2020) 15867–15881. DOI:10.1007/s10853-020-05122-3
- Graebe H., Netz A., Baesch S., Haerdtner V., Kwade A., A solvent-free electrode coating technique for all solid state lithium ion batteries, ECS Transactions, 77 (2017) 393–401. DOI:10.1149/07711.0393ecst
- Gratz E., Sa Q., Apelian D., Wang Y., A closed loop process for recycling spent lithium ion batteries, Journal of Power Sources, 262 (2014) 255–262. DOI:10.1016/j.jpowsour.2014.03.126
- Grießl D., Huber K., Scherbauer R., Kwade A., Dispersion kinetics of carbon black for the application in lithium-ion batteries, Advanced Powder Technology, 32 (2021) 2280–2288. DOI:10.1016/j.apt.2021.05.003
- Guo S.-h., Chen G., Peng J.-h., Chen J., Li D.-b., Liu L.-j., Microwave assisted grinding of ilmenite ore, Transactions of Nonferrous Metals Society of China, 21 (2011) 2122–2126. DOI:10.1016/S1003-6326(11)60983-7
- Haarmann M., Haselrieder W., Kwade A., Extrusion-based processing of cathodes: influence of solid content on suspension and electrode properties, Energy Technology, 8 (2020) 1801169. DOI:10.1002/ente.201801169
- Habib K., Hansdóttir S.T., Habib H., Critical metals for electromobility: Global demand scenarios for passenger vehicles, 2015–2050, Resources, Conservation and Recycling, 154 (2020) 104603. DOI:10.1016/j.resconrec.2019.104603
- Hanghofer I., Brinek M., Eisbacher S.L., Bitschnau B., Volck M., Hennige V., Hanzu I., Rettenwander D., Wilkening H.M.R., Substitutional disorder: structure and ion dynamics of the argyrodites Li6PS5Cl, Li6PS5Br and Li6PS5I, Physical Chemistry Chemical Physics, 21 (2019) 8489–8507. DOI:10.1039/C9CP00664H
- Hanisch C., Diekmann J., Stieger A., Haselrieder W., Kwade A., Recycling of lithium-ion batteries, in: Yan J. (Ed.), Handbook of Clean Energy Systems, John Wiley & Sons, Ltd, Chichester, UK, 2015a, pp. 1–24, ISBN: 9781118388587. DOI: 10.1002/9781118991978.hces221
- Hanisch C., Loellhoeffel T., Diekmann J., Markley K.J., Haselrieder W., Kwade A., Recycling of lithium-ion batteries: a novel method to separate coating and foil of electrodes, Journal of Cleaner Production, 108 (2015b)301–311. DOI: 10.1016/j.jclepro.2015.08.026
- Haselrieder W., Ivanov S., Tran H.Y., Theil S., Froböse L., Westphal B., Wohlfahrt-Mehrens M., Kwade A., Influence of formulation method and related processes on structural, electrical and electrochemical properties of LMS/NCA-blend electrodes, Progress in Solid State Chemistry, 42 (2014) 157–174. DOI:10.1016/j.progsolidstchem.2014.04.009
- Hashem A.M.A., Abdel-Ghany A.E., Eid A.E., Trottier J., Zaghib K., Mauger A., Julien C.M., Study of the surface modification of LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion battery, Journal of Power Sources, 196 (2011) 8632–8637. DOI:10.1016/j.jpowsour.2011.06.039
- Hayashi A., Masuzawa N., Yubuchi S., Tsuji F., Hotehama C., Sakuda A., Tatsumisago M., A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature, Nature communications, 10 (2019) 5266. DOI:10.1038/s41467-019-13178-2
- He S., Huang S., Wang S., Mizota I., Liu X., Hou X., Considering critical factors of silicon/graphite anode materials for practical high-energy lithium-ion battery applications, Energy & Fuels, 35 (2021) 944–964. DOI:10.1021/acs.energyfuels.0c02948
- Heck C.A., Horstig M.-W. von, Huttner F., Mayer J.K., Haselrieder W., Kwade A., Review—knowledge-based process design for high quality production of NCM811 cathodes, Journal of The Electrochemical Society, 167 (2020) 160521. DOI:10.1149/1945-7111/abcd11
- HMC (Hosokawa Micron Corporation), 2021, Faculty S series, F-S – spheronization, <https://www.hosokawamicron.co.jp/en/machines/181> accessed09122021.
- Hou X., Zhang M., Wang J., Hu S., Liu X., Shao Z., High yield and low-cost ball milling synthesis of nano-flake Si@SiO2 with small crystalline grains and abundant grain boundaries as a superior anode for Li-ion batteries, Journal of Alloys and Compounds, 639 (2015) 27–35. DOI:10.1016/j.jallcom.2015.03.127
- Hu R., Sun W., Chen Y., Zeng M., Zhu M., Silicon/graphene based nanocomposite anode: large-scale production and stable high capacity for lithium ion batteries, J. Mater. Chem. A, 2 (2014) 9118–9125. DOI:10.1039/c4ta01013b
- Huang W., Cheng Q., Qin X., Carbon nanotubes as a conductive additive in LiFePO4 cathode material for lithium-ion batteries, Russian Journal of Electrochemistry, 46 (2010) 175–179. DOI:10.1134/S1023193510020084
- Ibarra-Palos A., Strobel P., Darie C., Bacia M., Soupart J.B., Nanosized manganese oxide as cathode material for lithium batteries: Influence of carbon mixing and grinding on cyclability, Journal of Power Sources, 146 (2005) 294–299. DOI:10.1016/j.jpowsour.2005.03.093
- Janek J., Zeier W.G., A solid future for battery development, Nature Energy, 1 (2016) 1167. DOI:10.1038/nenergy.2016.141
- Jin Y., Zhu B., Lu Z., Liu N., Zhu J., Challenges and recent progress in the development of Si anodes for lithium-ion battery, Advanced Energy Materials, 7 (2017) 1700715. DOI:10.1002/aenm.201700715
- Ju S.H., Kang Y.C., Fine-sized LiNi0.8Co0.15Mn0.05O2 cathode powders prepared by combined process of gas-phase reaction and solid-state reaction methods, Journal of Power Sources, 178 (2008) 387–392. DOI:10.1016/j.jpowsour.2007.11.112
- Kang H.-C., Jun D.-K., Jin B., Jin E.M., Park K.-H., Gu H.-B., Kim K.-W., Optimized solid-state synthesis of LiFePO4 cathode materials using ball-milling, Journal of Power Sources, 179 (2008) 340–346. DOI:10.1016/j.jpowsour.2007.12.093
- Kato Y., Hori S., Saito T., Suzuki K., Hirayama M., Mitsui A., Yonemura M., Iba H., Kanno R., High-power all-solid-state batteries using sulfide superionic conductors, Nature Energy, 1 (2016) 16030. DOI:10.1038/nenergy.2016.30
- Kerman K., Luntz A., Viswanathan V., Chiang Y.-M., Chen Z., Review—practical challenges hindering the development of solid state li ion batteries, Journal of The Electrochemical Society, 164 (2017) A1731–A1744. DOI:10.1149/2.1571707jes
- Kim S.-B., Kim S.-J., Kim C.-H., Kim W.-S., Park K.-W., Nanostructure cathode materials prepared by high-energy ball milling method, Materials Letters, 65 (2011) 3313–3316. DOI:10.1016/j.matlet.2011.07.023
- Kingman S.W., Recent developments in microwave processing of minerals, International Materials Reviews, 51 (2006) 1–12. DOI:10.1179/174328006X79472
- Knieke C., Steinborn C., Romeis S., Peukert W., Breitung-Faes S., Kwade A., Nanoparticle production with stirred-media mills: opportunities and limits, Chemical Engineering & Technology, 33 (2010) 1401–1411. DOI:10.1002/ceat.201000105
- Kolmogorov A.N., Die lokale Struktur der Turbulenz in einer inkompressiblen zähen Flüssigkeit bei sehr großen Reynoldsschen Zahlen, Compt. Rend. Acad. Sc. USSR, 30 (1958).
- Kraft M.A., Ohno S., Zinkevich T., Koerver R., Culver S.P., Fuchs T., Senyshyn A., Indris S., Morgan B.J., Zeier W.G., Inducing high ionic conductivity in the lithium superionic argyrodites Li6+xP1–xGexS5I for all-solid-state batteries, Journal of the American Chemical Society, 140 (2018) 16330–16339. DOI:10.1021/jacs.8b10282
- Krauskopf T., Culver S.P., Zeier W.G., Local tetragonal structure of the cubic superionic conductor Na3PS4, Inorganic Chemistry, 57 (2018) 4739–4744. DOI:10.1021/acs.inorgchem.8b00458
- Krekel J., Zerkleinerung von agglomeraten in scherströmungen mit besonders hoher schubspannung, Chemie Ingenieur Technik, 38 (1966) 229–234. DOI:10.1002/cite.330380307
- Kremer L.S., Hoffmann A., Danner T., Hein S., Prifling B., Westhoff D., Dreer C., Latz A., Schmidt V., Wohlfahrt-Mehrens M., Manufacturing process for improved ultra-thick cathodes in high-energy lithium-ion batteries, Energy Technology, 8 (2020) 1900167. DOI:10.1002/ente.201900167
- Kudu Ö.U., Famprikis T., Fleutot B., Braida M.-D., Le Mercier T., Islam M.S., Masquelier C., A review of structural properties and synthesis methods of solid electrolyte materials in the Li2S – P2S5 binary system, Journal of Power Sources, 407 (2018) 31–43. DOI:10.1016/j.jpowsour.2018.10.037
- Kwade A., A stressing model for the description and optimization of grinding processes, Chemical Engineering & Technology, 26 (2003) 199–205. DOI:10.1002/ceat.200390029
- Kwade A., Hagelüken C., Kohl H., Buchert M., Herrmann C., Vahle T., vonWittken R., Carrara M., Daelemans S., Ehrenberg H., Fluchs S., Goldmann D. et al., Circular Economy Initiative Deutschland (Ed.), Resource-Efficient Battery Life Cycles – Driving Electric Mobility with the Circular Economy, acatech/SYSTEMIQ, Munich/London, 2020. DOI:10.48669/ceid_2021-2
- Kwade A., Haselrieder W., Leithoff R., Modlinger A., Dietrich F., Droeder K., Current status and challenges for automotive battery production technologies, Nature Energy, 3 (2018) 290–300. DOI:10.1038/s41560-018-0130-3
- Kwade A., Diekmann J., Recycling of Lithium-Ion Batteries, Springer, Cham, 2018, ISBN: 9783319705712. DOI:10.1007/978-3-319-70572-9
- Kwak H., Han D., Lyoo J., Park J., Jung S.H., Han Y., Kwon G., Kim H., Hong S.-T., Nam K.-W., Jung Y.S., New cost-effective halide solid electrolytes for all-solid-state batteries: mechanochemically prepared Fe3+-substituted Li2ZrCl6, Advanced Energy Materials, 11 (2021) 2003190. DOI:10.1002/aenm.202003190
- Kwon H.-J., Woo S.-W., Lee Y.-J., Kim J.-Y., Lee S.-M., Achieving high-performance spherical natural graphite anode through a modified carbon coating for lithium-ion batteries, Energies, 14 (2021) 1946. DOI:10.3390/en14071946
- Lai J., Guo H., Wang Z., Li X., Zhang X., Wu F., Yue P., Preparation and characterization of flake graphite/silicon/carbon spherical composite as anode materials for lithium-ion batteries, Journal of Alloys and Compounds, 530 (2012) 30–35. DOI:10.1016/j.jallcom.2012.03.096
- Lämmerer W., Flachberger H., Wissenswertes zur charakterisierung und aufbereitung von rohgrafiten, BHM Berg- und Hüttenmännische Monatshefte, 162 (2017) 336–344. DOI:10.1007/s00501-017-0651-2
- Landesfeind J., Eldiven A., Gasteiger H.A., Influence of the binder on lithium ion battery electrode tortuosity and performance, Journal of The Electrochemical Society, 165 (2018) A1122–A1128. DOI:10.1149/2.0971805jes
- Landi B.J., Ganter M.J., Cress C.D., DiLeo R.A., Raffaelle R.P., Carbon nanotubes for lithium ion batteries, Energy & Environmental Science, 2 (2009) 638. DOI:10.1039/b904116h
- Leblanc D., Hovington P., Kim C., Guerfi A., Bélanger D., Zaghib K., Silicon as anode for high-energy lithium ion batteries: From molten ingot to nanoparticles, Journal of Power Sources, 299 (2015) 529–536. DOI:10.1016/j.jpowsour.2015.09.040
- Lee J., Extraction of lithium from lepidolite using mixed grinding with sodium sulfide followed by water leaching, Minerals, 5 (2015) 737–743. DOI:10.3390/min5040521
- Li H., Rao W., Gu Z., Wang Y., Wang N., Lv X., Chen B., Jiang H., Chen L., Nano-grinding derived high-performance Li1.2Ni0.13Co0.13Mn0.54O2 cathode material: from kilogram-scale synthesis to its pouch cell, Ionics, 27 (2021) 491–506. DOI:10.1007/s11581-020-03875-0
- Li M., Hou X., Sha Y., Wang J., Hu S., Liu X., Shao Z., Facile spray-drying/pyrolysis synthesis of core–shell structure graphite/silicon-porous carbon composite as a superior anode for Li-ion batteries, Journal of Power Sources, 248 (2014) 721–728. DOI:10.1016/j.jpowsour.2013.10.012
- Li X., Liang J., Luo J., Banis M.N., Wang C., Li W., Deng S., Yu C., Zhao F., Hu Y., Sham T.-K., Zhang L., Zhao S., Lu S., Huang H., Li R., Adair K.R., Sun X., Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries, Energy & Environmental Science, 12 (2019) 2665–2671. DOI:10.1039/C9EE02311A
- Li X., Liang J., Yang X., Adair K.R., Wang C., Zhao F., Sun X., Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries, Energy & Environmental Science, 13 (2020) 1429–1461. DOI:10.1039/C9EE03828K
- Liu H., Cheng X., Chong Y., Yuan H., Huang J.-Q., Zhang Q., Advanced electrode processing of lithium ion batteries: a review of powder technology in battery fabrication, Particuology, 57 (2021) 56–71. DOI:10.1016/j.partic.2020.12.003
- Liu N., Lu Z., Zhao J., McDowell M.T., Lee H.-W., Zhao W., Cui Y., A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes, Nature Nanotechnology, 9 (2014) 187–192. DOI:10.1038/nnano.2014.6
- Liu X.H., Huang J.Y., In situ TEM electrochemistry of anode materials in lithium ion batteries, Energy & Environmental Science, 4 (2011) 3844. DOI:10.1039/c1ee01918j
- Liu X.H., Zhong L., Huang S., Mao S.X., Zhu T., Huang J.Y., Size-dependent fracture of silicon nanoparticles during lithiation, ACS nano, 6 (2012) 1522–1531. DOI:10.1021/nn204476h
- Liu Y., Liu H., Zhao X., Wang L., Liang G., Effect of spherical particle size on the electrochemical properties of lithium iron phosphate, Journal of Wuhan University of Technology-Mater. Sci. Ed., 34 (2019) 549–557. DOI:10.1007/s11595-019-2086-y
- Lu J., Peng Q., Wang W., Nan C., Li L., Li Y., Nanoscale coating of LiMO2 (M = Ni, Co, Mn) nanobelts with Li+-conductive Li2TiO3: toward better rate capabilities for Li-ion batteries, Journal of the American Chemical Society, 135 (2013) 1649–1652. DOI:10.1021/ja308717z
- Martha S.K., Sclar H., Szmuk Framowitz Z., Kovacheva D., Saliyski N., Gofer Y., Sharon P., Golik E., Markovsky B., Aurbach D., A comparative study of electrodes comprising nanometric and submicron particles of LiNi0.50Mn0.50O2, LiNi0.33Mn0.33Co0.33O2, and LiNi0.40Mn0.40Co0.20O2 layered compounds, Journal of Power Sources, 189 (2009) 248–255. DOI:10.1016/j.jpowsour.2008.09.090.
- Mathieux F., Ardente F., Bobba S., Nuss P., Blengini G., Alves Dias P., Blagoeva D., Torres De Matos C., Wittmer D., Pavel C., Hamor T., Saveyn H., Gawlik B., Orveillon G., Huygens D. et al., Critical Raw Materials and the Circular Economy – Background Report, EUR 28832 EN, Publications Office of the European Union, Luxembourg, 2017, ISBN 978-92-79-74283-5. DOI:10.2760/378123
- Mayer J.K., Almar L., Asylbekov E., Haselrieder W., Kwade A., Weber A., Nirschl H., Influence of the carbon black dispersing process on the microstructure and performance of Li-ion battery cathodes, Energy Technology, 8 (2020) 1900161. DOI:10.1002/ente.201900161
- Moskvitch K., Field of dreams: the plants that love heavy metal, New Scientist, 221 (2014) 46–49. DOI:10.1016/S0262-4079(14)60594-7
- Müller J., Abdollahifar M., Vinograd A., Nöske M., Nowak C., Chang S.-J., Placke T., Haselrieder W., Winter M., Kwade A., Wu N.-L., Si-on-graphite fabricated by fluidized bed process for high-capacity anodes of Li-ion batteries, Chemical Engineering Journal, 407 (2021) 126603. DOI:10.1016/j.cej.2020.126603
- Müller S., Eller J., Ebner M., Burns C., Dahn J., Wood V., Quantifying inhomogeneity of lithium ion battery electrodes and its influence on electrochemical performance, Journal of The Electrochemical Society, 165 (2018) A339–A344. DOI:10.1149/2.0311802jes
- Mundszinger M., Farsi S., Rapp M., Golla-Schindler U., Kaiser U., Wachtler M., Morphology and texture of spheroidized natural and synthetic graphites, Carbon, 111 (2017) 764–773. DOI:10.1016/j.carbon.2016.10.060
- Nagao M., Hayashi A., Tatsumisago M., High-capacity Li2S–nanocarbon composite electrode for all-solid-state rechargeable lithium batteries, Journal of Materials Chemistry, 22 (2012) 10015–10020. DOI:10.1039/C2JM16802B
- Naghash A.R., Lee J.Y., Lithium nickel oxyfluoride (Li1–zNi1+zFyO2–y) and lithium magnesium nickel oxide (Li1–z(MgxNi1–x)1+zO2) cathodes for lithium rechargeable batteries, Electrochimica Acta, 46 (2001) 2293–2304. DOI:10.1016/S0013-4686(01)00452-2
- Nakamura H., Kawaguchi T., Masuyama T., Sakuda A., Saito T., Kuratani K., Ohsaki S., Watano S., Dry coating of active material particles with sulfide solid electrolytes for an all-solid-state lithium battery, Journal of Power Sources, 448 (2020) 227579. DOI:10.1016/j.jpowsour.2019.227579
- Natarajan C., Fujimoto H., Mabuchi A., Tokumitsu K., Kasuh T., Effect of mechanical milling of graphite powder on lithium intercalation properties, Journal of Power Sources, 92 (2001) 187–192. DOI:10.1016/S0378-7753(00)00528-0
- Ng S.H., Vix-Guterl C., Bernardo P., Tran N., Ufheil J., Buqa H., Dentzer J., Gadiou R., Spahr M.E., Goers D., Novák P., Correlations between surface properties of graphite and the first cycle specific charge loss in lithium-ion batteries, Carbon, 47 (2009) 705–712. DOI:10.1016/j.carbon.2008.11.008
- Nguyen H., Banerjee A., Wang X., Tan D., Wu E.A., Doux J.-M., Stephens R., Verbist G., Meng Y.S., Single-step synthesis of highly conductive Na3PS4 solid electrolyte for sodium all solid-state batteries, Journal of Power Sources, 435 (2019) 126623. DOI:10.1016/j.jpowsour.2019.05.031
- Nilssen B.E., Henriksen B.R., Kleiv R.A., Effect of operation parameters and formulation on the submicron and nano size silicon powder properties produced by mechanical grinding, Silicon for the Chemical and Solar Industry XV, (2020) 295–308.
- Nilssen B.E., Kleiv R.A., Silicon powder properties produced in a planetary ball mill as a function of grinding time, Grinding Bead Size and Rotational Speed, Silicon, 12 (2020) 2413–2423. DOI:10.1007/s12633-019-00340-0
- Nöske M., Breitung-Faes S., Kwade A., Electrostatic stabilization and characterization of fine ground silicon particles in ethanol, Silicon, 11 (2019) 3001–3010. DOI:10.1007/s12633-019-0089-0
- Obrovac M.N., Zheng L., Garayt M.D.L., Engineered particle synthesis by dry particle microgranulation, Cell Reports Physical Science, 1 (2020) 100063. DOI:10.1016/j.xcrp.2020.100063
- Ohno S., Koerver R., Dewald G., Rosenbach C., Titscher P., Steckermeier D., Kwade A., Janek J., Zeier W.G., Observation of chemomechanical failure and the influence of cutoff potentials in all-solid-state Li–S batteries, Chemistry of Materials, 31 (2019) 2930–2940. DOI:10.1021/acs.chemmater.9b00282
- Palaniandy S., Powell M., Hilden M., Allen J., Kermanshahi K., Oats B., Lollback M., VertiMill® – preparing the feed within floatable regime at lower specific energy, Minerals Engineering, 73 (2015) 44–52. DOI:10.1016/j.mineng.2014.11.014
- Pan T., Alvarado J., Zhu J., Yue Y., Xin H.L., Nordlund D., Lin F., Doeff M.M., Structural degradation of layered cathode materials in lithium-ion batteries induced by ball milling, Journal of The Electrochemical Society, 166 (2019) A1964–A1971. DOI:10.1149/2.0091910jes
- Park B.H., Jeong J.H., Lee G.-W., Kim Y.-H., Roh K.C., Kim K.-B., Highly conductive carbon nanotube micro-spherical network for high-rate silicon anode, Journal of Power Sources, 394 (2018) 94–101. DOI:10.1016/j.jpowsour.2018.04.112
- Parker T., Shi F., Evans C., Powell M., The effects of electrical comminution on the mineral liberation and surface chemistry of a porphyry copper ore, Minerals Engineering, 82 (2015) 101–106. DOI:10.1016/j.mineng.2015.03.019
- Peng Z.S., Wan C.R., Jiang C.Y., Synthesis by sol–gel process and characterization of LiCoO2 cathode materials, Journal of Power Sources, 72 (1998) 215–220. DOI:10.1016/S0378-7753(97)02689-X
- Peters J.F., Baumann M., Zimmermann B., Braun J., Weil M., The environmental impact of Li-Ion batteries and the role of key parameters – a review, Renewable and Sustainable Energy Reviews, 67 (2017) 491–506. DOI:10.1016/j.rser.2016.08.039
- Powell M.S., How our clients will drive the need to slash energy use in mining. Proc. 26th IMCET Int. Mining Congress, Antalya, Turkey, 16–19 April 2019.
- Qi C., Ma X., Ning G., Song X., Chen B., Lan X., Li Y., Zhang X., Gao J., Aqueous slurry of S-doped carbon nanotubes as conductive additive for lithium ion batteries, Carbon, 92 (2015) 245–253. DOI:10.1016/j.carbon.2015.04.028
- Rao R.P., Adams S., Studies of lithium argyrodite solid electrolytes for all-solid-state batteries, Physica Status Solidi (A), 208 (2011) 1804–1807. DOI:10.1002/pssa.201001117
- Reindl A., Aldabergenova S., Altin E., Frank G., Peukert W., Dispersing silicon nanoparticles in a stirred media mill-investigating the evolution of morphology, structure and oxide formation, Physica Status Solidi (A), 204 (2007) 2329–2338. DOI:10.1002/pssa.200622557
- Reindl A., Voronov A., Gorle P.K., Rauscher M., Roosen A., Peukert W., Dispersing and stabilizing silicon nanoparticles in a low-epsilon medium, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 320 (2008) 183–188. DOI:10.1016/j.colsurfa.2008.01.045
- Schlem R., Banik A., Ohno S., Suard E., Zeier W.G., Insights into the lithium sub-structure of superionic conductors Li3YCl6 and Li3YBr, Chemistry of Materials, 33 (2021a)327–337. DOI:10.1021/acs.chemmater.0c04352
- Schlem R., Muy S., Prinz N., Banik A., Shao-Horn Y., Zobel M., Zeier W.G., Mechanochemical synthesis: a tool to tune cation site disorder and ionic transp6ort properties of Li3MCl6 (M = Y, Er) superionic conductors, Advanced Energy Materials, 10 (2020) 1903719. DOI:10.1002/aenm.201903719
- Schlem R., Burmeister C.F., Michalowski P., Ohno S., Dewald G.F., Kwade A., Zeier W.G., Energy storage materials for solid-state batteries: design by mechanochemistry, Advanced Energy Materials, 11 (2021b) 2101022. DOI:10.1002/aenm.202101022
- Schönert K., A first survey of grinding with high-compression roller mills, International Journal of Mineral Processing, 22 (1988) 401–412. DOI:10.1016/0301-7516(88)90075-0
- Schunemann J.-H., Dreger H., Bockholt H., Kwade A., Smart electrode processing for battery cost reduction, ECS Transactions, 73 (2016) 153–159. DOI:10.1149/07301.0153ecst
- Seeba J., Reuber S., Heubner C., Müller-Köhn A., Wolter M., Michaelis A., Extrusion-based fabrication of electrodes for high-energy Li-ion batteries, Chemical Engineering Journal, 402 (2020) 125551. DOI:10.1016/j.cej.2020.125551
- Shaw-Stewart J., Alvarez-Reguera A., Greszta A., Marco J., Masood M., Sommerville R., Kendrick E., Aqueous solution discharge of cylindrical lithium-ion cells, Sustainable Materials and Technologies, 22 (2019) e00110. DOI:10.1016/j.susmat.2019.e00110
- Sinha N.N., The effect of particle size on performance of cathode materials of Li-ion batteries, Journal of the Indian Institute of Science, 89 (2009) 381–392.
- Sitando O., Crouse P.L., Processing of a Zimbabwean petalite to obtain lithium carbonate, International Journal of Mineral Processing, 102–103 (2012) 45–50. DOI:10.1016/j.minpro.2011.09.014
- Stehmann F., Bradtmöller C., Scholl S., Separation of the electrolyte—thermal drying, in: Kwade A., Diekmann J. (Eds.), Recycling of Lithium-Ion Batteries. Sustainable Production, Life Cycle Engineering and Management, Springer, Cham., 2018, pp. 139–153, ISBN: 978-3-319-70571-2. DOI:10.1007/978-3-319-70572-9_8
- Stein M. IV, Chen C.-F., Mullings M., Jaime D., Zaleski A., Mukherjee P.P., Rhodes C.P., Probing the effect of high energy ball milling on the structure and properties of LiNi1/3Mn1/3Co1/3O2 cathodes for Li-ion batteries, Journal of Electrochemical Energy Conversion and Storage, 13 (2016) 031001. DOI:10.1115/1.4034755
- Steinrötter M., Carbon based anodes – a rare earth situation? Third German Electric Vehicle Congress, Bonn, Germany, 2011.
- Stöffler H., Zinkevich T., Yavuz M., Hansen A.-L., Knapp M., Bednarčík J., Randau S., Richter F.H., Janek J., Ehrenberg H., Indris S., Amorphous versus crystalline Li3PS4: local structural changes during synthesis and li ion mobility, The Journal of Physical Chemistry C, 123 (2019) 10280–10290. DOI:10.1021/acs.jpcc.9b01425
- Sui D., Xie Y., Zhao W., Zhang H., Zhou Y., Qin X., Ma Y., Yang Y., Chen Y., A high-performance ternary Si composite anode material with crystal graphite core and amorphous carbon shell, Journal of Power Sources, 384 (2018) 328–333. DOI:10.1016/j.jpowsour.2018.03.008
- Sun F., Markötter H., Dong K., Manke I., Hilger A., Kardjilov N., Banhart J., Investigation of failure mechanisms in silicon based half cells during the first cycle by micro X-ray tomography and radiography, Journal of Power Sources, 321 (2016a)174–184. DOI:10.1016/j.jpowsour.2016.04.126
- Sun W., Hu R., Zhang M., Liu J., Zhu M., Binding of carbon coated nano-silicon in graphene sheets by wet ball-milling and pyrolysis as high performance anodes for lithium-ion batteries, Journal of Power Sources, 318 (2016b)113–120. DOI: 10.1016/j.jpowsour.2016.04.016
- Švrček V., Rehspringer J.-L., Gaffet E., Slaoui A., Muller J.-C., Unaggregated silicon nanocrystals obtained by ball milling, Journal of Crystal Growth, 275 (2005) 589–597. DOI:10.1016/j.jcrysgro.2004.12.012
- Szczech J.R., Jin S., Nanostructured silicon for high capacity lithium battery anodes, Energy & Environmental Science, 4 (2011) 56–72. DOI:10.1039/c0ee00281j
- Takeuchi S., Suzuki K., Hirayama M., Kanno R., Sodium superionic conduction in tetragonal Na3PS4, Journal of Solid State Chemistry, 265 (2018) 353–358. DOI:10.1016/j.jssc.2018.06.023
- Talens Peiró L., Villalba Méndez G., Ayres R.U., Lithium: sources, production, uses, and recovery outlook, JOM, 65 (2013) 986–996. DOI:10.1007/s11837-013-0666-4
- Tatsumisago M., Hama S., Hayashi A., Morimoto H., Minami T., New lithium ion conducting glass-ceramics prepared from mechanochemical Li2S–P2S5 glasses, Solid State Ionics, 154–155 (2002) 635–640. DOI:10.1016/S0167-2738(02)00509-X
- Taylor L., Skuse D., Blackburn S., Greenwood R., Stirred media mills in the mining industry: material grindability, energy-size relationships, and operating conditions, Powder Technology, 369 (2020) 1–16. DOI:10.1016/j.powtec.2020.04.057
- Utsunomiya T., Hatozaki O., Yoshimoto N., Egashira M., Morita M., Influence of particle size on the self-discharge behavior of graphite electrodes in lithium-ion batteries, Journal of Power Sources, 196 (2011) 8675–8682. DOI:10.1016/j.jpowsour.2011.06.070
- Velázquez-Martínez O., Valio J., Santasalo-Aarnio A., Reuter M., Serna-Guerrero R., A critical review of lithium-ion battery recycling processes from a circular economy perspective, Batteries, 5 (2019) 68. DOI:10.3390/batteries5040068
- Wang H., Ikeda T., Fukuda K., Yoshio M., Effect of milling on the electrochemical performance of natural graphite as an anode material for lithium-ion battery, Journal of Power Sources, 83 (1999) 141–147. DOI:10.1016/S0378-7753(99)00288-8
- Wenzel V., Moeller R.S., Nirschl H., Influence of mixing technology and the potential to modify the morphological properties of materials used in the manufacture of lithium-ion batteries, Energy Technology, 2 (2014) 176–182. DOI:10.1002/ente.201300091
- Wenzel V., Nirschl H., Nötzel D., Challenges in lithium-ion-battery slurry preparation and potential of modifying electrode structures by different mixing processes, Energy Technology, 3 (2015) 692–698. DOI:10.1002/ente.201402218
- Wills B.A., Napier-Munn T., Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th Edition, Butterworth-Heinemann, Amsterdam, London, 2006, ISBN: 9780080479477.
- Wu H., Chan G., Choi J.W., Ryu I., Yao Y., McDowell M.T., Lee S.W., Jackson A., Yang Y., Hu L., Cui Y., Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control, Nature Nanotechnology, 7 (2012) 310–315. DOI:10.1038/nnano.2012.35
- Wu Y.S., Yeh T.S., Lee Y.H., Lee Y.C., Spheroidization modification of artificial graphite applied as anode materials for high rate lithium ion batteries, Advanced Materials Research, 201–203 (2011) 421–424. DOI:10.4028/www.scientific.net/AMR.201-203.421
- Xu C., Dai Q., Gaines L., Hu M., Tukker A., Steubing B., Future material demand for automotive lithium-based batteries, Communications Materials, 1 (2020) 99. DOI:10.1038/s43246-020-00095-x
- Yan Q., Li X., Wang Z., Wu X., Wang J., Guo H., Hu Q., Peng W., Extraction of lithium from lepidolite by sulfation roasting and water leaching, International Journal of Mineral Processing, 110–111 (2012) 1–5. DOI:10.1016/j.minpro.2012.03.005
- Yazami R., Touzain P., A reversible graphite-lithium negative electrode for electrochemical generators, Journal of Power Sources, 9 (1983) 365–371. DOI:10.1016/0378-7753(83)87040-2
- Yoon S., Kim H., Oh S.M., Surface modification of graphite by coke coating for reduction of initial irreversible capacity in lithium secondary batteries, Journal of Power Sources, 94 (2001) 68–73. DOI:10.1016/S0378-7753(00)00601-7
- Yudha C.S., Muzayanha S.U., Widiyandari H., Iskandar F., Sutopo W., Purwanto A., Synthesis of LiNi0.85Co0.14Al0.01O2 cathode material and its performance in an NCA/graphite full-battery, Energies, 12 (2019) 1886. DOI:10.3390/en12101886
- Zeng X., Li J., Singh N., Recycling of spent lithium-ion battery: a critical review, Critical Reviews in Environmental Science and Technology, 44 (2014) 1129–1165. DOI:10.1080/10643389.2013.763578
- Zhang C., Gu L., Kaskhedikar N., Cui G., Maier J., Preparation of silicon@silicon oxide core-shell nanowires from a silica precursor toward a high energy density Li-ion battery anode, ACS Applied Materials & Interfaces, 5 (2013a)12340–12345. DOI: 10.1021/am402930b
- Zhang Q., Wang X., Lu W., Tang F., Guo J., Yu W., Qu M., Yu Z., Comparisons of short carbon nanotubes containing conductive additives of cathode for lithium ion batteries, Materials Research Bulletin, 48 (2013b)2865–2870. DOI: 10.1016/j.materresbull.2013.04.010
- Zhang Z., Shao Y., Lotsch B., Hu Y.-S., Li H., Janek J., Nan C., Nazar L., Maier J., Armand M., Chen L., New horizons for inorganic solid state ion conductors, Energy & Environmental Science, 11 (2018) 1945–1976. DOI:10.1039/C8EE01053F
- Zhang Y., Cao H., Zhang J., Xia B., Synthesis of LiNi0.6Co0.2Mn0.2O2 cathode material by a carbonate co-precipitation method and its electrochemical characterization, Solid State Ionics, 177 (2006) 3303–3307. DOI:10.1016/j.ssi.2006.09.008
- Zhao M., Li B.-Q., Zhang X.-Q., Huang J.-Q., Zhang Q., A perspective toward practical lithium-sulfur batteries, ACS Central Science, 6 (2020) 1095–1104. DOI:10.1021/acscentsci.0c00449
- Zhao N., Li Y., Zhao X., Zhi X., Liang G., Effect of particle size and purity on the low temperature electrochemical performance of LiFePO4/C cathode material, Journal of Alloys and Compounds, 683 (2016) 123–132. DOI:10.1016/j.jallcom.2016.04.070
Nomenclature
Al
Aluminum
AM
Active material
ASSB
All-solid-state battery
BEV
Battery electric vehicle
BMS
Battery management system
Br
Bromine
CAM
Cathode active material
CB
Carbon black
CEID
Circular Economy Initiative Germany
CG
Conductive graphite
CMC
Carboxymethyl cellulose
CNT
Carbon nano tubes
CO2
Carbon dioxide
DMC
Dimethyl carbonate
EC
Ethylene carbonate
ELV
End of life vehicle
EMC
Ethyl methyl carbonate
Em,slurry
Specific energy related to slurry mass
EoL
End of life
EU
European Union
Gr
Graphite
HF
Hydrogen fluoride
HPGR
High pressure grinding rolls
LFP
Lithium iron phosphate
LMNO
Lithium manganese nickel oxide
Li
Lithium
LIB
Lithium ion batteries
LiFePO3
Lithium iron phosphate
LIS
Lithium sulfur battery
LLZO
Lithium lanthanum zirconium oxide
LPS
Li3PS4 (sulfidic solid electrolyte)
Mio.
Million
NCA
Lithium nickel cobalt aluminum oxide
NiMH
Nickel metal hydrate
NMC
Lithium nickel manganese cobalt oxide (LiNi1–y–zMnyCozO2)
NMP
N-2-methyl-pyrrolidone
PEO
Polyethylene oxide
PHEV
Plug-in hybrid electric vehicle
PVDF
Polyvinylidene fluoride
SAG
Semi-autogenous grinding
SBR
Styrene-butadiene rubber
SEI
Solid electrolyte interface
Si@Gr
Graphite with Si-nanoparticles
Si@GrC
Graphite with Si-nanoparticles and carbon coating tph tons per hour
VGCF
Vapor grown carbon fiber
wt
weight
x50,3
Volumetric median particle size
σion
ionic conductivity
Authors’ Short Biographies
Arno Kwade

Arno Kwade worked 9 years as a process engineer in leading positions in industry after finishing his doctorate in 1996. Since 2005 he is leading as Professor the Institute for Particle Technology at TU Braunschweig. His research focus lies on developing process-structure-property relationships for processes in which particles are mechanically stressed and formulated, from milling and mechanochemical synthesis over mixing to production and calendaring of electrodes for lithium-ion and all-solid-state batteries. Today he is Chairman of the Battery LabFactory Braunschweig (BLB) and the Center of Pharmaceutical Engineering (PVZ).
Marcel Möller

Marcel Möller received his master‘s degree in biochemical and chemical engineering at the TU Braunschweig in 2019. After a year of industrial experience at Volkswagen AG as engineer for battery slurry production, he joined the Institute for Particle Technology. In his research, he targets high quality and low-cost production of nano-sized silicon via wet-grinding. For that purpose, he develops different formulating strategies as well as suited process conditions and evaluates the impact on the production of the silicon nanoparticles.
Jannes Müller

Jannes Müller got his master’s degree at the Technical University Braunschweig in 2018 and is working as a PhD student in the working group Battery Process Engineering at the Institute for Particle Technology since then. His research focuses on the scalable preparation of nano-silicon graphite composites as anode material for lithium-ion batteries. In his recent publication he demonstrated the preparation via fluidized bed granulation with subsequent carbon coating enabling high performance silicon materials.
Jutta Hesselbach

Jutta Hesselbach studied mechanical engineering at the TU Braunschweig, where she proceeded her studies in the field of process engineering as a research associate at the Institute for Particle Technology. Within this field, she completed her doctoral thesis in July 2020 at the TU Braunschweig. Now she works at the Fraunhofer Institute for Surface Engineering and Thin Films IST within the department Process and Production Engineering for Sustainable Energy Storage Systems, where she was promoted to Head of the Group “Material and Process Development”.
Sabrina Zellmer

Sabrina Zellmer studied mechanical engineering at the TU Braunschweig, where she proceeded her studies in the field of nanomaterials as a research associate at the Institute for Particle Technology. She completed her doctoral studies in the field of particle functionalization and spray drying. During her work at the iPAT, she broadened her scientific expertise by working in the BLB, becoming the head of the junior research group “Solid-State Battery Materials and Electrodes”. Since January 2019 Sabrina Zellmer is Head of Department Process and Production Engineering for Sustainable Energy Storage Systems at the Fraunhofer IST.
Stefan Doose

Stefan Doose received his master degree in biotechnology with a focus on process engineering from TU Braunschweig in 2017. Subsequently, he started his work as a research assistant (PhD student) in the Battery Process Engineering research group at the Institute of Particle Technology of Technische Universtität Braunschweig in 2017 with a focus on mechanical safety studies with accompanying online gas analysis as well as mechanical-thermal recycling processes of lithium-ion batteries.
Julian Mayer

Julian Mayer received his Bachelor of Engineering from the University of Applied Sciences Munich in 2013 and subsequently completed his Master’s degree from the Technical University of Braunschweig in 2016. Since then, he has been researching the continuous and discontinuous dispersion of lithium-ion battery slurries, with focus on the carbon black fragmentation at the Institute for Particle Technology. Since 2020, he has been deputy head of the Battery Process Engineering group.
Peter Michalowski

Peter Michalowski studied physics at the University Jena and graduated in the field of superconductivity. Subsequently, he worked on his doctoral thesis project on cathodes for lithium ion batteries at the University Oldenburg and received his title in 2017. He joined the Institute for Particle Technology at the TU Braunschweig in 2018 and became leader of the junior research group “Solid-State Battery Materials and Electrodes” in 2019. Since the start of 2021 he has been the head of the battery processing division of the iPAT and part of the head office of the Battery LabFactory Braunschweig.
Malcolm Powell

Malcolm Powell has applied fundamental comminution research to design and process improvement on over 60 mines worldwide during 35 years at Mintek, UCT, and then as Professor of comminution at the JKMRC in Australia. Malcolm collaborates extensively, with close compatriots on 5 continents forming the Global Comminution Collaborative (GCC)—providing an expert research and consulting. Malcolm’s research vision is of integrated total process simulation as a tool for innovation—linking geology, mining, energy and size reduction, gangue rejection and recovery into flexible process design and process optimization.
Sandra Breitung-Faes

Sandra Breitung-Faes studied process engineering at TU Clausthal and received her doctoral grade in 2009 at the Institute for Particle Technology at the TU Braunschweig with the topic of wet stirred media milling of ceramic nano particles. Afterwards she had the position of a team leader for grinding, a group leader for grinding and dispersing and is now, since 2018 the division manager for powder and slurry processes at the Institute for Particle Technology of TU Braunschweig.
 https://orcid.org/0000-0002-6348-7309
https://orcid.org/0000-0002-6348-7309
 https://ror.org/010nsgg66 Fraunhofer Institute for Surface Engineering and Thin Films IST, Germany [ドイツ]
https://ror.org/010nsgg66 Fraunhofer Institute for Surface Engineering and Thin Films IST, Germany [ドイツ]  https://ror.org/048vzmn57
https://ror.org/048vzmn57
 https://ror.org/010nsgg66
https://ror.org/010nsgg66
 https://ror.org/010nsgg66
https://ror.org/010nsgg66
 https://ror.org/048vzmn57
https://ror.org/048vzmn57
 https://ror.org/048vzmn57
https://ror.org/048vzmn57
 https://ror.org/010nsgg66
https://ror.org/010nsgg66
 https://ror.org/010nsgg66
https://ror.org/010nsgg66
 https://ror.org/010nsgg66
https://ror.org/010nsgg66
 https://ror.org/00rqy9422
https://ror.org/00rqy9422
 https://ror.org/010nsgg66
https://ror.org/010nsgg66




















