2023 年 40 巻 p. 197-218
2023 年 40 巻 p. 197-218
The development of energy storage devices providing high energy and power densities and long-term stability will play an important role in the future utilization of sustainable energy sources. Numerous efforts have been devoted to achieving these requirements, especially the design of advanced electrode materials. For this reason, there is growing interest in the innovation of new carbon-based materials with enhanced electrochemical performance. Nanostructured carbon spheres (CSs) have attracted significant attention due to their prominent properties, such as high surface area, excellent electrical conductivity, tunable porosity, and surface functionality. This review offers a comprehensive overview into the recent advances of nanostructured CSs within the last five years, focusing on synthetic strategies for producing carbon particles with precisely controlled morphologies and interior structures, as well as the potential applications of these particles as high-performance electrode materials in rechargeable batteries and supercapacitors. The challenges and perspectives on future research directions are highlighted, focusing on the controlled synthesis and functionalization of nanostructured CSs with tunable structures and properties that are well-suited to practical applications. This review is intended to serve as a helpful resource to researchers involved in the fabrication of new CS materials and the development of methods to control their structure and morphology.
The greenhouse effect is widely recognized as one of the main factors responsible for global climate change, which has begun to affect our daily life and has become a significant concern affecting the existence of all living species (Zhang et al., 2022; Tanno and Makino, 2018). Therefore, the development of renewable and green energy technologies for a sustainable future, which could mitigate the most severe impacts of climate change, is a highly desirable goal. Until now, numerous extensive research efforts are focused on the design of low-cost and environment-friendly energy storage devices with high electrochemical performance characteristics, including rechargeable batteries and supercapacitors (Long et al., 2017; Zhang M. et al., 2018). The electrode materials in these electrochemical energy storage devices play a crucial role, which determine their overall performance. Consequently, the exploration of multifunctional electrode materials is still a hot and vital topic.
In order to meet these goals described above, the key aspects are the innovative design of nanostructured particulate materials and the discovery of fabrication processes that allow for the synthesis of materials having precisely tailored morphologies, structures and chemical characteristics. It will also be necessary to search for greener and more cost-effective synthetic processes to allow the widespread utilization of such devices. Interestingly, natural materials often tend to adopt spherical shapes because this morphology minimizes surface energy and is highly stable. From an engineering viewpoint, spherical particles also offer many advantages because they have no sharp edges and are thus less likely to be damaged during processing. In addition, the size and regular shape of spheres allow for the effective ordering of subjects in densely packed states, especially when necks and pores are present. Finally, spheres provide excellent fluidity (Cao et al., 2021b; Tian et al., 2019). As a consequence of these advantages, carbon-based spheres have captivated vast attention due to the combined advantages of carbon materials with spherical colloids. These materials can exhibit several unique features, including regular geometry, high uniformity, good fluidity, superior packing density, and tunable particle size distribution (Liu et al., 2015; Tian et al., 2019). Despite the enormous achievements made in many fields, carbon spheres (CSs) continue to emerge and fabricate from smart synthetic strategies. These intriguing advantages and exciting prospects have stimulated worldwide research activities of CSs, particularly in energy-related applications. Therefore, the development and innovation of advanced CSs having well-defined structural and surface characteristics are expected to play a critical role in the energy field, which provide an opportunity to improve the performance in energy storage devices with both high power and energy densities.
Although several review articles concerning carbon-based materials (e.g., mesoporous carbon, carbon nanotubes, graphene) have been published over the last decade (Benzigar et al., 2018; Kerdnawee et al., 2017; Tian et al., 2019; Zhang M. et al., 2018; Zhao et al., 2019), the scope of the review covers the critical advances of the nanostructured CSs, focusing on the recent progress in the fabrication with precisely controlled structures and functionalities through smart synthetic strategies. Such strategies are necessary to achieve better performance in energy storage devices. On this basis, the aim of the present review was to offer an overview into the fabrication techniques of CSs, with a particular emphasis on new and promising developments. As a critical point, a comprehensive overview is provided that discusses the most up-to-date strategies (those devised primarily within the last five years) for the rational synthesis of CSs with diverse structures. In addition, the utilization of such materials as key components in a wide range of energy storage applications is explored. Fig. 1 provides a diagram summarizing various aspects of the nanostructured CSs considered herein. These aspects comprise fabrication processes and carbon sources, various methods for the tuning of morphology and architecture, functionalization, and applications in energy storage devices. To highlight the above-mentioned important point, the present review is organized as follows. Firstly, introducing the promising approaches for the design and synthesis of CSs using three routes (e.g., template-free, soft templating, and hard templating) by combining the excellent work from worldwide researchers. Secondly, overviewing the performance of CSs with various structures and their composites in energy storage devices (e.g., rechargeable batteries and supercapacitors). Furthermore, there is still a lack of a clear discussion regarding the specific relationship between carbon structures and electrochemical performance characteristics. As a matter of fact, the correlation between the synthesis strategy, structure, and characteristics of CSs would allow these materials to be used more extensively in energy storage devices; therefore, their preparation-structure-function relevance is expected to be understood. Finally, offering some concluding remarks on the current challenges and perspectives related to nanostructured CS materials, as well as highlighting the promising future progress in the field of energy-related devices.

A comprehensive overview of the preparation and functionalization of nanostructured CSs for energy storage applications.
Various structures of CS materials can be obtained using numerous synthesis processes. Fig. 2 shows typical schemes for preparing nanostructured CSs with different strategies. These synthesis processes can generally be classified into three categories: template-free, soft template and hard template approaches, which will be highlighted in this section.

Synthetic strategies for the preparation of CSs using (a) template-free, (b) soft template, and (c) hard template approaches.
The large-scale production of CSs will require processes that are simple, energy efficient, environmentally friendly, and cost-effective. On this basis, the development of template-free approaches capable of producing CSs having a wide range of pore sizes would be preferable, as shown in Fig. 2(a). Three simple and commonly used template-free synthetic strategies, including hydrothermal carbonization (HTC) method, extension of the Stöber method, and aerosol-assisted method will be investigated. Common precursor materials for the fabrication of CSs in conjunction with template-free strategies and the respective physical properties of the obtained CSs are summarized in Table 1. Recently, numerous review articles has been carefully explained the synthesis of nanostructured CSs using HTC and extension of the Stöber methods (Liu et al., 2015; Tian et al., 2019). Therefore, in this section, we mainly focused on the fabrication of nanostructured CSs using aerosol-assisted techniques.
Summary of the conditions used for the template-free synthesis of functionalized CSs, the textural parameters of these materials and their potential applications.
| No. | Material | Process | Precursor | Structure | dpore (nm) | SSA (m2/g) | Vtotal (cm3/g) | Applications | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | N-ACSs a | HTC b + Activation | Glucose | Abundant microporous structure | 1.6 | 1579 | 0.66 | LSBs c | (Xiang et al., 2018) |
| 2 | N-CSs | Surfactant-assisted HTC | Glucose | Microporous structure | < 1.5 | 844 | 0.35 | LIBs d and SIBs e | (Zhang H. et al., 2019) |
| 3 | ACSs | HTC + Activation | Xylose | Micro-mesoporous structure | — | 2675 | 1.62 | Supercapacitors | (Sun et al., 2020) |
| 4 | PCSs f | HTC | Starch | Hierarchical porous structure with coexistence of micro- and mesopores | 2–3 | 393–973 | 0.18–0.27 | Supercapacitors | (Lu et al., 2018) |
| 5 | PCSs | HTC + Activation | Starch | Porous structure with high content of micropores and sufficient mesopores | 3.9 | 1973 | 0.89 | Supercapacitors and SIBs | (Zhang J. et al., 2020) |
| 6 | PCSs | HTC + Activation | GAML g | Porous structure with dominant micropores and low content of mesopores | 1.8–2.1 | 1746–2478 | 0.87–1.24 | Supercapacitors | (Hao et al., 2018) |
| 7 | N-CSs | Extended the Stöber method | 3-Aminophenol and formaldehyde | Microporous structure | < 2 | 61–356 | — | PIBs h | (Wang S. et al., 2020) |
| 8 | N-HCSs i | Extended the Stöber method | Resorcinol and formaldehyde | Micro-/mesoporous structure. Different inner structures (solid, yolk-shell, hollow) can be obtained depending on the changed polymerization degree of internal RF resin | — | 946 | 0.51 | Supercapacitors | (Zhang L. et al., 2019) |
| N-YS-CSs j | — | 1263 | 0.68 | ||||||
| N-SCSs k | — | 501 | 0.29 | ||||||
| 9 | Pomegranate-like CSs | Spray drying + Activation | 3-Aminophenol and formaldehyde | Pomegranate-like structure and rich packing voids with abundant micropores | — | 1477 | 0.83 | Supercapacitors | (Feng et al., 2019) |
| 10 | CSs | Spray drying + Carbonization | Kraft lignin | Dense structure with abundant micropores and small amounts of mesopores | 0.7 | 936–1233 | 0.31–0.44 | — | (Cao et al., 2021b) |
| 11 | HCSs | Spray drying + Carbonization | Kraft lignin | Porous hollow structure composed of micro- and mesopores | 0.7, 2.4 | 1536–2425 | 0.75–1.57 | Supercapacitors | (Cao et al., 2021a) |
| 12 | PCSs | Spray drying + Carbonization | Kraft lignin | By changing the NaOH concentration, the structure can be adjusted from a compact to hollow structure | 0.7, 2.4, 20–100 | 761–1513 | 0.34–1.01 | Supercapacitors | (Kitamoto et al., 2022) |
| 13 | HPCSs l | Spray drying + Carbonization | Sodium lignosulfonate | Hierarchical porous hollow structure comprised micro-, meso- and macropores | 1–7, 10–100 | 963–1342 | 0.72–1.05 | Supercapacitors | (Pang et al., 2018b) |
| 14 | MCSs m | Spray drying + Carbonization | Chitosan | Mesoporous structure with unique bimodal pore size distribution | ~ 4, 7 | 645–1292 | 0.33–1.29 | LSBs | (Zhou H. et al., 2018) |
| 15 | PCSs | Spray pyrolysis | Coal | Hierarchical porous structure composed of micro- and mesopores | 1.7–2.5 | 547–948 | 0.23–0.52 | Supercapacitors | (Guo et al., 2017) |
| 16 | N,S-PCSs n | Spray pyrolysis | Coal | Porous structure composed of micro- and mesopores | — | 589–635 | 0.38–0.43 | Supercapacitors | (Lv et al., 2020) |
| 17 | PCSs | Spray pyrolysis + Activation | Glucose | Honeycomb-like hierarchical porous structure including micro-, meso-, and macropores | < 1, 1–5, 100 | 1443–1837 | 1.23–1.40 | Supercapacitors | (Tang et al., 2018) |
| 18 | N-HCSs | Spray pyrolysis + Carbonization | Glucose and glucosamine | Hollow structure with large number of mesopores within the shell | — | 327 | 0.13 | Supercapacitors | (Qu et al., 2018) |
Aerosol-assisted techniques, such as spray drying and spray pyrolysis, are cost-effective, simple, continuous and scalable methods for the preparation of CSs (Ogi et al., 2014; Debecker et al., 2018; Leng et al., 2019; Gradon et al., 2020). Because of these advantages, such methods are frequently used to produce dry powders in the food, pharmaceutical, and chemical industries. Generally, depending on the operating conditions, solid or hollow spherical particles can be produced in the spray process (Cao et al., 2021b; 2021a; Nguyen et al., 2021; 2022). Feng et al. prepared pomegranate-like carbon microspheres (PCSs) by fabricating monodisperse, submicron CSs via a scalable template-free spray drying-assisted approach (Feng et al., 2019). In this process, monodisperse 3-aminophenol/formaldehyde (3-AF) resin spheres were initially synthesized based on the polymerization of 3-aminophenol and formaldehyde in deionized water without the use of a catalyst. The monodisperse resin colloids were subsequently spray dried to generate pomegranate-like resin microspheres during the spray drying process. Finally, after carbonization and potassium hydroxide (KOH) activation, the pomegranate-like resin microspheres were converted into PCSs. These PCSs comprised well-defined microspheres with a high specific surface area (SSA) of 1477 m2/g and a relatively broad size distribution ranging from 1 μm to 5 μm.
Until now, considering the sustainable development of CS materials, the use of biomass as a precursor has received increasing attention due to its renewability, reproducibility, readily accessible, and environmental friendliness. Although biomass has been utilized as a raw material for the fabrication of carbon materials, there are few reports on the successful production of CSs from biomass-based materials using template-free spray drying method. Cao et al. has successfully fabricated spherical carbon particles using a template-free spray drying approach followed by the carbonization process, utilizing KOH as the activator and Kraft lignin as the carbon precursor (Fig. 3(a)) (Cao et al., 2021a; 2021b). Adjusting the KOH concentration in the reaction solution was found to allow tuning of the CS structure from dense to hollow, as shown in the TEM images (Fig. 3(b–d)). A possible mechanism for the formation of carbon particles is also studied in their research, with the aim of providing a more in-depth understanding of the process. In addition, hollow carbon spheres (HCSs) having a high SSA of 2424.8 m2/g along with a micro-mesoporous structure were obtained at KOH-to-lignin mass ratios below 1.5. Very recently, the same group has proposed a fundamental modification of the fabrication process of porous CSs by employing sodium hydroxide (NaOH) as the substitute activation agent in place of the more conventionally used KOH (Kitamoto et al., 2022). NaOH offers some advantages over KOH in terms of less corrosiveness, low cost, and simple handling procedure, all of which are appealing particularly from an industrial point of view. The results indicate that the structure of carbon particles can be tuned from a compact to hollow structure, and the surface textural characteristics can be easily adjusted by changing the amount of NaOH. The significant achievements and ongoing efforts in this field suggest that these processes will allow the development of advanced carbon materials and the high value-added utilization of Kraft lignin as a promising material for potential applications.

(a) An illustration of the synthesis of CSs from Kraft lignin via a spray drying process; Transmission electron microscopy (TEM) images of carbon particles generated using KOH/lignin mass ratios of (b) 0.17, (c) 0.67, and (d) 1.33.
In the spray pyrolysis process, droplets of the liquid precursor are formed via ultrasonication (He and Wang, 2019; Ogi et al., 2020; Septiani et al., 2021; 2022; Le et al., 2022) and promptly carbonized at high temperatures under the protection of inert gases (Balgis et al., 2015; 2017). The size and morphology of the resulting CSs can be adjusted by changing the reaction conditions, reactant concentration ratios, and carbonization temperature. In recent years, there have been several publications regarding the synthesis of CSs using a template-free spray pyrolysis process. Guo et al. developed a novel strategy for producing hierarchical porous CSs by utilizing coal as the raw material in conjunction with ultrasonic spray pyrolysis (Guo et al., 2017). In this process, the raw coal is chemically oxidized by a highly concentrated mixture of sulfuric acid and nitric acid, such that numerous functional groups are introduced, including −C=O, −OH, −NH2, −SO3H, and −NO2. Subsequently, the as-prepared coal oxide can be readily dissolved in an alkaline solution to obtain a transparent precursor solution. The pyrolysis temperature was found to have a significant effect on the pore architecture and SSA of the CSs generated using this process. As the temperature was increased from 700 to 1000 °C, micro-/mesoporous structures were observed in these CSs with the SSA and total pore volume of the samples rapidly increasing from 547.2 to 948.5 m2/g and from 0.23 to 0.52 cm3/g, respectively. To further improve the electrochemical properties of carbon materials, the same group was developed multiple heteroatoms N/S doping porous CSs through ultrasonic spray pyrolysis method employing l-Cysteine as nitrogen and sulfur precursor along with coal as the carbon precursor (Lv et al., 2020). The results reveal that the homogeneous doping of N and S elements not only improves the hydrophilicity of the CSs but also provides more active sites, resulting in an increase in specific capacitance.
2.2 Soft template-assisted liquid phase/aerosol processSoft templates are typically organic molecules, macromolecules or supramolecules that are easily decomposed at relatively low carbonization temperature (below 400 °C). The synthesis of porous CSs using the soft template typical involves several key steps (shown in Fig. 2(b)): (i) the dissolution of the template molecules and carbon precursor in a suitable solvent, (ii) the soft templates turn into micelles during solvent evaporation and simultaneous interaction of these micelles with the carbon precursor via hydrogen bonding as well as hydrophobic, hydrophilic or electrostatic interactions, and (iii) the heat treatment of the precursor/template composite in an inert atmosphere with complete decomposition of the soft template. Various carbon precursors and soft templates that are frequently used in the preparation of CSs, as well as the physical properties of the resulting materials, are summarized in Table 2.
Summary of the conditions used for the soft template synthesis of functionalized CSs, the textural parameters of these materials and their potential applications.
| No. | Material | Process | Template | Precursor | Structure | dpore (nm) | SSA (m2/g) | Vtotal (cm3/g) | Applications | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | N-MCSs a | Soft template + Activation | PS-b-PEO b | Dopamine | Interconnected hierarchical porous structure (micro- and mesopores). Mesopores are well-distributed on the sphere surface | 4.2–16.0 | 397–2320 | 0.29–1.41 | Supercapacitors | (Tang et al., 2017) |
| 2 | N-MCSs | Soft template | PS-b-PAA c | Dopamine | Core-shell structure with abundant mesopores in the core | 14.8 | 449 | 0.60 | SIBs | (Mao et al., 2020) |
| 3 | N-MCSs | Soft template + Pyrolysis | Pluronic F127 | 2-Aminophenol and formaldehyde | Mesoporous structure with uniform mesopore size (5 nm) | 5 | 439 | 0.33 | Supercapacitors | (Wang et al., 2018) |
| 4 | PCSs d | Soft template | Pluronic F127 | Larch sawdust (biomass) | Hierarchical porous structure consisting of interconnected and worm-like pores | — | 659–760 | 0.44–0.67 | Supercapacitors | (Song et al., 2019) |
| 5 | N,O-CSs e | Soft template + HTC | Pluronic F127 | 3-Aminophenol and formaldehyde | Hierarchical micro-/mesoporous structure with interconnected and worm-like pores | 0.6, 2–5 | 2012–3203 | 0.99–1.93 | Supercapacitors | (Liu S. et al., 2016) |
| 6 | N-MCSs | Soft template | Pluronic F127 | Melamine and formaldehyde | Porous structure composed of large mesopores | 1.2, 6.7–8.1 | 791–883 | 1.3–1.5 | SIBs | (Guo et al., 2021) |
| 7 | N-PCSs | Soft template + HTC | Pluronic F108 | Phenol and formaldehyde | Porous structure with many micropores and few mesopores | — | 1481 | 0.90 | Supercapacitors | (Liang et al., 2019) |
| 8 | YS-CSs f | Soft template | CTAB g | Resorcinol and formaldehyde | Hierarchical porous yolk-shell structure | 1.5, 2–10 | 543–703 | 0.59–1.47 | PIBs | (Zhang H. et al., 2018) |
| 9 | N,O-YS-CSs h | Soft template + HTC | CTAB | Resorcinol and formaldehyde | Yolk-shell structure comprised dense core and porous shell layer (micro- and mesopores) | — | 440–996 | — | PIBs | (Chong et al., 2021) |
| 10 | N-HMCSs | Soft template (Extended the Stöber method) | CTAC i | Resorcinol and formaldehyde | By changing amount of EDA, mesoporous yolk-shell and hollow structures are obtained | 2.4 | 2001 | 1.86 | Supercapacitors | (Liu C. et al., 2016) |
| N-YS-MCSs | 2.6 | 1169 | 0.88 | |||||||
| 11 | HCSs | Soft template (Extended the Stöber method) | CTAB | Phenol and formaldehyde | Increasing EtOH/H2O ratio, the structures were ad-justed from irregular to hollow to core-shell. Abundant mesopores under appropriate EtOH/H2O ratio |
2.9 | 544–1065 | 0.57–1.54 | Supercapacitors | (Du et al., 2020) |
| 12 | APCSs | Soft template | DDAB j | 3-Aminophenol and formaldehyde | Hollow structure with interconnected pores | — | 259–1746 | 0.50–1.94 | Supercapacitors | (Li et al., 2021) |
| 13 | N-PCSs | Soft template + HTC | [C12mim]Br k | 3-Aminophenol and amphiphilic aliphatic aldehydes | Hierarchical porous structure including micro- and mesopores. | 3.7–6.3, 25 | 812–1040 | 0.64–1.54 | Supercapacitors | (Jia et al., 2022) |
| 14 | N-MCHSs | Soft template + HTC | Gemini l | 3-Aminophenol and formaldehyde | Interconnected porous hollow structure (meso- and macropores) | < 2, 5–20, 20–110 | 1215–1517 | 1.12–3.22 | Supercapacitors | (Li et al., 2020) |
Over the past decade, there has been significant process in the direct synthesis of mesoporous CSs from amphiphilic molecules such as surfactant or block copolymer self-assembles with carbon precursor, which is stabilized by thermal treatment. Different carbon precursors, such as phenolic resin or their derivatives, carbohydrates, nitrogen and carbon-rich compounds (e.g., dopamine), and natural polymers (e.g., cellulose, chitin and lignin) are commonly used for the fabrication of mesoporous CSs. Tang et al. prepared porous CSs with sub-micrometer particle size (about 300 nm) using the linear block copolymer PS-b-PEO as the soft template and dopamine as the carbon source in conjunction with KOH activation (Tang et al., 2017). The size of mesopores could be precisely tuned from 4 to 16 nm by using PS-b-PEO copolymers having different molecular weights as pore forming agents in this process. Despite such prior work, the fabrication of mesoporous CSs with large pore size from soft templates as a means of providing efficient pathways for electrolyte diffusion remains a great challenge. On this basis, Fei et al. explored the fabrication of nitrogen-doped mesoporous CSs having ultra-large mesopore sizes (up to 24.5 nm) by utilizing PS-b-PEO brush block copolymers (BBCPs) along with a small molecule surfactant (Pluronic F127) as the template and dopamine as the carbon and nitrogen source followed by carbonization and thermal decomposition of the polymer templates, as illustrated in Fig. 4(a) (Fei et al., 2020). Mesoporous CSs with bimodal mesopore size distributions (having apexes at 6.5 and 24.5 nm) were confirmed by the peak in the dV/logD curve (red line curve) and the cumulative pore volume is calculated by summing individual pore volumes (black line curve), as shown in Fig. 4(b). Specifically, BBCPs corresponded to the large mesopores and the Pluronic F127 template was responsible for the small mesopores. This work demonstrated a unique and adaptable approach for synthesizing CSs with a precisely tailored morphology and bimodal pore sizes for potential applications in energy storage. Peng et al. reported the fabrication of highly uniform mesoporous CSs with adjustable pore sizes and structures (e.g., smooth, golf ball, multi-chambered, and dendritic nanospheres) (Fig. 4(c)) (Peng et al., 2019). In this work, Pluronic F127 was utilized as a soft template, dopamine was employed as a carbon and nitrogen source, and organic molecules (e.g., 1,3,5-trimethylbenzene) played a critical role in the evolution of pore size and also affected the interfacial interactions between the Pluronic F127 and dopamine. The CSs produced by this process had an ultra-large pore size of 37 nm, small average particle size of 128 nm, high SSA of 635 m2/g, and high nitrogen content of 6.8 wt%.

(a) An illustration showing the fabrication of monomodal and bimodal porous CSs through the self-assembly of dopamine, PS-b-PEO BBCPs, and Pluronic F127; (b) pore size distribution of mesoporous CSs. Reprinted with permission from Ref. (Fei et al., 2020). Copyright 2020, American Chemical Society. (c) An illustration showing the formation of N-doped mesoporous carbon nanospheres corresponded to TEM images with different morphologies and mesostructures using a versatile nano-emulsion assembly method. Reprinted with permission from Ref. (Peng et al., 2019). Copyright 2019, American Chemical Society.
In some studies, ordered mesoporous CSs have been fabricated by a simple one-step process. Song et al. synthesized hierarchical porous CSs using Pluronic F127 as a soft template and biomass (e.g., larch sawdust) as the carbon precursor via a spray pyrolysis method (Song et al., 2019). The morphology, particle size, and porosity of these CSs could be simply adjusted by varying the amount of F127 in the reaction system. The obtained porous CSs were found to have a high SSA up to 760.3 m2/g and mesopore sizes that could be adjusted within the range of 10–40 nm. In addition to block polymers, surfactants such as Triton X-100 have also been employed as the soft template to synthesize HCSs (Zhou et al., 2017). The HCSs obtained from this process had a SSA of approximately 893.3 m2/g and a total pore volume of 0.76 cm3/g.
Yolk-shell CSs with a unique core@void@shell structure have attracted tremendous interests as a consequence of their hierarchical pore architectures that are present in a controlled arrangement within each single particle (Zhang H. et al., 2018). Liu et al. prepared nitrogen-doped yolk-shell mesoporous CSs via a soft template approach, employing ethylenediamine (EDA) as both a nitrogen source and a base catalyst in a Stöber-silica/carbon assembly system (Liu C. et al., 2016). The resulting nitrogen-doped yolk-shell mesoporous CSs possessed a uniform mesopore size of 2.6 nm and a high SSA of 2001 m2/g. Shu et al. designed nitrogen-doped yolk-shell CSs with tunable mesoporous surfaces and particle sizes based on a soft template approach (Shu et al., 2018). The fabrication process used RF resin as the carbon precursor, melamine as the nitrogen precursor, cetyltrimethylammonium bromide (CTAB) as the pore-forming template, TEOS as the skeleton structure and ammonia as the catalyst. Because the rate of hydrolysis of TEOS was slower than the rate of polymerization of melanin resorcinol formaldehyde (MRF) in the initial stage of this process, a CTAB/MRF formed as the core and SiO2/CTAB/MRF formed as the shell. The subsequent carbonization of these particles at a high temperature produced a hollow core-shell structure and nitrogen-doped yolk-shell CSs were obtained after the SiO2 template was removed using an NaOH solution.
2.3 Hard template-assisted liquid phase/aerosol processThe hard template approach, also known as the nanocasting approach, is commonly used materials with relatively rigid structures to guide the growth of materials based on providing limited space (Fuji et al., 2013; Li et al., 2016; Cao et al., 2020) such as SiO2 (Wang H. et al., 2020), polystyrene latex (PSL) (Arif et al., 2016), poly(methyl methacrylate) (PMMA) (Ye et al., 2016) and calcium carbonate (CaCO3) (Ni et al., 2019). Several key processing steps are involved in the preparation of CSs using a hard template strategy, as shown in Fig. 2(c). These include (i) the fabrication of sphere-shaped hard template materials, (ii) the impregnation of these hard templates with a carbon precursor, (iii) the carbonization of the resulting carbon precursor/template composite in an inert atmosphere at a high temperature, and (iv) the removal of the hard template via thermal decomposition or chemical etching methods, depending on the nature of the hard template. One of the key factors influencing the architecture and pore distribution is the dispersion of the hard template in the precursor solution. The resulting materials will possess rich pores with good connectivity if the template is well-dispersed in the precursor. Therefore, developing a method for enhancing the dispersion of hard template in the precursor solution is highly desirable (Ogi et al., 2017). Various CSs having different textural features have been prepared using different hard templates and the details of the materials and structural parameters are provided in Table 3.
Summary of the conditions used for the hard template synthesis of functionalized CSs, the textural parameters of these materials and their potential applications.
| No. | Material | Process | Template | Precursor | Structure | dpore (nm) | SSA (m2/g) | Vtotal (cm3/g) | Applications | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | UHCSs a | Hard template | SiO2 sphere | PTCDA b | Hollow structure with non-porous shells, ultrathin shell thickness | — | 10 | 0.12 | LSBs | (Song J. et al., 2018) |
| 2 | N,P-HPCSs c | Hard template | SiO2 sphere | Dopamine | Hollow structure with abundant micropores | 0.5–2 | 185–677 | 0.31–1.64 | SIBs | (Wang H. et al., 2020) |
| 3 | N-HPCSs | Hard template + Activation | SiO2 sphere | Dopamine | Hollow structure with abundant ultramicropores and reasonable supermicro- and mesopores | 0.5, 0.8, 1.3, 2.7 | 860–1789 | 0.63–1.18 | Supercapacitors | (Song Z. et al., 2018) |
| 4 | N-HMCSs d | Hard template | SiO2 sphere | Dopamine | Hollow structure with abundant mesopores | 9.1 | 457 | 1.04 | LSBs | (Zhang et al., 2015) |
| 5 | N,P-PCSs e | Hard template | SiO2 sphere | Melamine and formaldehyde | Hollow sphere with interconnected micro-, meso-, macroporous structure | 2.6, 3.7 | 563–720 | 0.44–0.56 | Supercapacitors | (Zhang et al., 2017) |
| 6 | N-HCSs | Hard template | SiO2 sphere | m-Phenylenediamine and formaldehyde | Hollow structure (shell thickness ~25 nm). Micropores exist in the shell, while meso-/macropores correspond to the hollow cavity | < 2, 20–70 | 2044 | 1.60 | Supercapacitors | (Xu et al., 2021) |
| 7 | N,P,S-PCSs f | Hard template | SiO2 sphere | Aniline | Hierarchical porous structure with numerous pores within spheres | 0.5–1, 2–4, 32 | 358–1258 | 0.68–1.96 | Supercapacitors | (Yan et al., 2018) |
| 8 | HPCSs | Hard template | SiO2 sphere | Furfuryl alcohol | Mesoporous structure with macro-hollow core | 2–4 | 2489 | 1.45 | Supercapacitors | (Zhou M. et al., 2018) |
| 9 | N-HMCSs | Hard template | SiO2 sphere | PS/PAN g | Hollow structure Mesopores in the shells |
4.1 | 807 | 0.87 | Supercapacitors | (Chen et al., 2016b) |
| 10 | N-HCSs | Hard template | PS sphere | 3-Aminophenol and formaldehyde | Hollow structure with micro-mesopores in the shells | — | 365–772 | — | Supercapacitors | (Arif et al., 2016) |
| 11 | Hierarchical PCSs | Hard template | PS sphere | Phenolic resin | Hollow structure: macropores covered by a thin carbon shell Porous structure: macropores exposed on the surface | 2.8, 128 | 70–96 | — | — | (Balgis et al., 2015) |
| 12 | N,O-HCSs | Hard template + Activation | PS sphere | Pyrrole | Hierarchical hollow porous structure with abundant micro- and mesopores | 0.5–15 | 558–1036 | 0.66–1.01 | Supercapacitors | (Chen et al., 2017) |
| 13 | S,N-HCSs h | Hard template | PMMA sphere | Resorcinol and formaldehyde | Hollow spheres with microporous structure | — | 486 | — | SIBs | (Ye et al., 2016) |
| 14 | S,N-MHCSs | Hard template | CaCO3 sphere | Dopamine | Interconnected hollow sphere structure with mesopores | 5, 25 | 397 | — | SIBs | (Ni et al., 2019) |
| 15 | N-HCSs | Hard template | Cu2O sphere | 3-Aminophenol and formaldehyde | Micro-/mesoporous hollow structure with thin carbon shells (15–84 nm) | 1.2–1.5 | 19–112 | 0.07–0.34 | Supercapacitors | (Zhang D. et al., 2020) |
SiO2 spheres have been extensively applied as hard template to prepare CSs because they are inexpensive, have easily controllable sizes ranging from nanometer to micrometer and exhibit remarkable thermal stability together with a high degree of uniformity. Solid SiO2 spheres are usually fabricated via the Stöber method, based on the hydrolysis of a SiO2 precursor in a mixture of water and alcohol with ammonia as the catalyst (Stöber and Fink, 1968). HCSs can be obtained by coating of different carbon precursors on the surface of these SiO2 particles followed by carbonization and etching process. Wang et al. synthesized N-doped HCSs using a simple interfacial sol-gel coating process, utilizing colloidal SiO2 as the template, resorcinol/formaldehyde as the carbon precursor and EDA as both the nitrogen precursor and base catalyst (Wang et al., 2017). These HCSs possessed a uniform size of approximately 120 nm in diameter along with porous shells as thin as 10 nm. Density functional theory has demonstrated that N-doping could change the binding sites and enhance the adsorption of PF6 − ions in the carbon matrix. Zhang et al. developed a simple one-pot approach for synthesizing mesoporous HCSs with large controllable pore size based on a surfactant-free system (Zhang et al., 2016). Using a Stöber process, the reaction between resorcinol, formaldehyde and a TEOS/tetrapropyl orthosilicate (TPOS) mixture generated SiO2@SiO2/RF spheres without the addition of a cationic surfactant. In comparison with traditional TEOS-based system, the incorporation of TPOS, which undergoes hydrolysis and condensation rate more slowly than TEOS, provided better control over the formation of SiO2 core and primary particles. The pore sizes in the mesoporous HCSs could be precisely tuned over the range from micropores to a size of 13.9 nm by adjusting the TEOS/TPOS or ethanol/water ratio.
PSL spheres with uniform sizes and good dispersion have been widely used as hard template, partly because these materials are readily removed by thermal annealing at 400 °C (Arif et al., 2016) or dissolution using organic solvents (Chen et al., 2016a). Balgis et al. synthesized nanostructured carbon particles via ultrasonic spray pyrolysis based on the self-assembly behavior of phenolic resin and PSL particles (Balgis et al., 2014; 2015). By adjusting the repulsive or attractive forces between the phenolic resin and PSL particles, the morphology of the prepared carbon particles can be precisely controlled. Strong electrostatic attraction between the highly positively charged PSL and phenolic resin resulted in hollow carbon particles (Fig. 5(a)), whereas the electrostatic repulsion occurred in the presence of negatively charged PSL formed porous carbon particles (Fig. 5(b)). The mechanism has been proposed in Fig. 2(c) and the detailed explanations are demonstrated in Videos S1 and S2 (available publicly at https://doi.org/10.50931/data.kona.21014023). Furthermore, the fabrication of hierarchical bimodal macroporous carbon nanospheres with interconnected pores (with applications as a catalyst support) using a spray pyrolysis technique with phenolic resin as the carbon source and PSL spheres as the template was also introduced, as shown in Fig. 5(c–d) (Balgis et al., 2017). The morphology of the resulting carbon particles was greatly influenced by changing the size of PSL particles, as shown in the SEM and TEM images (Fig. 5(e–j)). A bimodal macroporous structure can enhance the surface area available to accommodate Pt loading without sacrificing active site accessibility. As a means of improving the electron-transfer characteristics of carbon, Arif et al. fabricated HCSs having high-nitrogen content based on microwave-assisted polymerization together with carbonization process, using PSL as the template and 3-aminophenol as the carbon source (Arif et al., 2016). The plausible mechanism of shell formation and the chemical bond between PSL particles and 3-aminophenol during the nucleation process are demonstrated in Fig. 5(k). By altering the 3-aminophenol/PSL mass ratio, the carbon shell thickness could be precisely adjusted from 14.2 to 66.6 nm, while the particle size could be easily controlled from 58.2 to 320 nm by changing the PSL particle size (Fig. 5(l–n)).
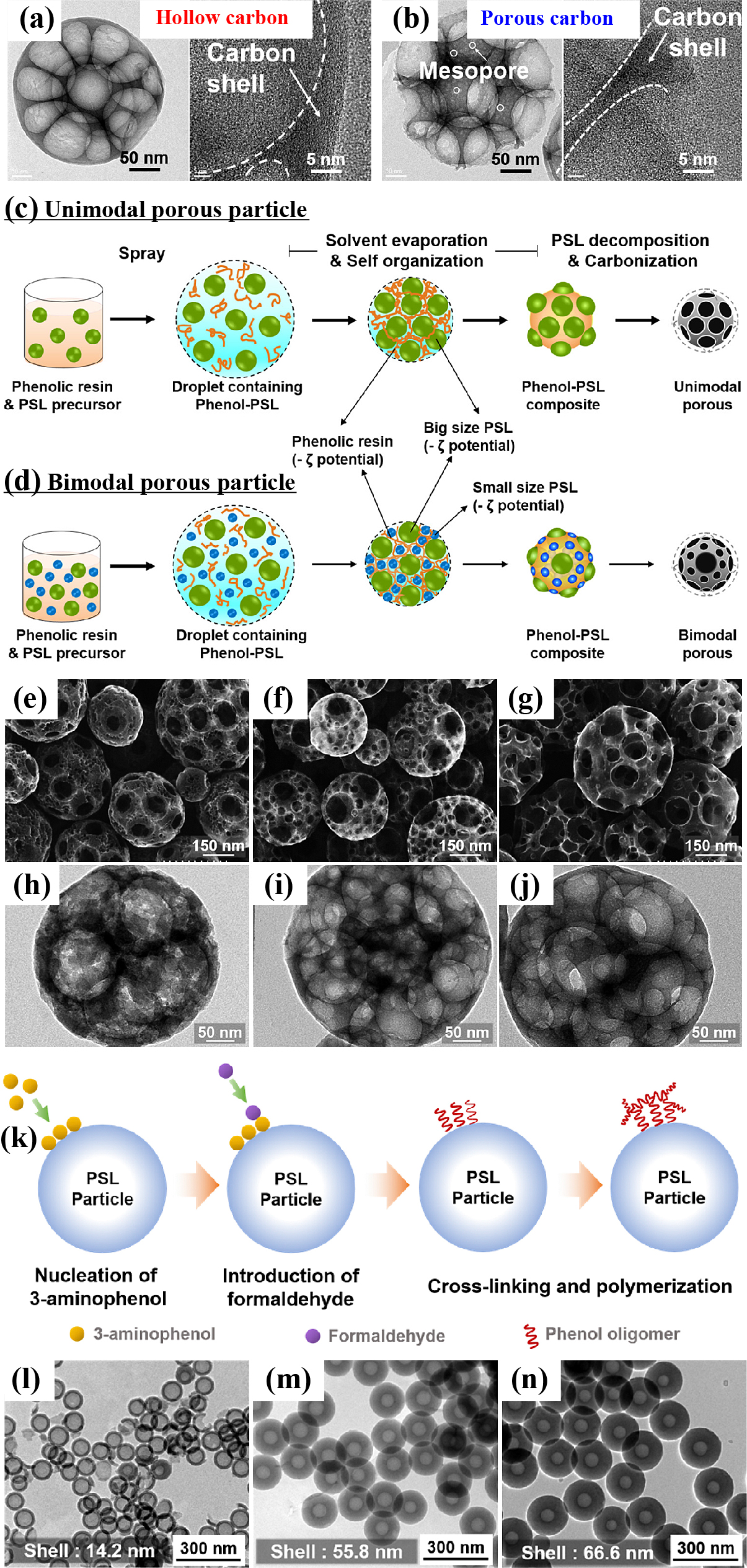
TEM images of carbon particle formation prepared via spray pyrolysis with (a) hollow CSs (using positively charged PSL), the video is available publicly at https://doi.org/10.50931/data.kona.21014023 and (b) porous CSs (using negatively charged PSL), the video is available publicly at https://doi.org/10.50931/data.kona.21014023. Reprinted with permission from Ref. (Balgis et al., 2014). Copyright 2014, American Chemical Society. Illustrations showing the formation of (c) unimodal and (d) bimodal macroporous CSs, SEM and TEM images of bimodal porous CSs made using PSL particle sizes of (e, h) 230 and 40 nm; (f, i) 230 and 90 nm; and (g, j) 230 and 120 nm. Reprinted with permission from Ref. (Balgis et al., 2017). Copyright 2017, American Chemical Society. (k) An illustration of the shell growth on the surface of PSL particles, TEM images of HCSs prepared at 3-aminophenol/PSL mass ratio of (l) 1, (m) 10, and (n) 15 with 63 nm PSL particles in each case. Reprinted with permission from (Arif et al., 2016b). Copyright 2016, Elsevier.
Another approach for producing porous CSs is by dissolution of the PSL using organic solvents. Chen et al. proposed a strategy for synthesizing mesoporous HCSs based on the “dissolution-capture” approach (Chen et al., 2016a). Polystyrene (PS) spheres serving as the template were prepared by emulsion polymerization and then coated with mesoporous SiO2. The PS cores were subsequently dissolved in tetrahydrofuran to form hollow cavities and was captured by the mesopores of the SiO2 shells. The as-captured PS was then crosslinked based on Friedel-Crafts alkylation and was utilized as the carbon source. After carbonization and etching to remove SiO2, mesoporous HCSs with a uniform mesopore size (4.1 nm) were obtained.
With the rapid development of electric vehicles and portable electronics, there have been increasing demands for the development of lithium-ion batteries (LIBs) having improved energy density, longer cycle life and faster charging rate. Graphite has traditionally been used as an anode material but provides a limited theoretical capacity (372 mAh/g) and a rate capability that cannot satisfy the requirements for enhanced performance, especially in the case of novel sodium-ion batteries (SIBs) or potassium-ion batteries (PIBs). Therefore, the development of new carbon-based materials for battery electrodes is critical. CSs are of particular interest among the variety of promising carbon materials because their small particle sizes and highly exposed surfaces ensure reduced charge transfer resistance during metalation/de-metalation. In addition, an isotropic spherical structure ensures a homogeneous distribution of current flow and allows a highly packed particle arrangement, resulting in higher volumetric energy densities. When employing CSs as an anode material for metal-ion batteries, large SSA, high pore volume, and good conductivity are crucial to obtaining the required metal-ion storage capacity and stability. Specifically, a high SSA provides for good contact with the electrolyte to sustain an elevated metal-ion flux through the interface, a large pore volume allows for metal ion and volume changes during metalation, while good conductivity ensures efficient electron transport.
The capacities and rate performances of anodes based on CSs have been significantly improved using two principal strategies. One such strategy involves doping single or dual heteroatoms (e.g., O, N, B, S, P) into carbon materials to modulate the electronic and chemical structures. Such modifications can lead to enlarged interlayer distance, enhanced electronic conductivity, improved metal ion/ electrolyte absorption and more active sites such that metal ion storage and cycling performance are enhanced (Long et al., 2017). In addition, the incorporation of such atoms modifies the pore structure. A hierarchical structure is desirable because micropores promote the reversible intercalation/de-intercalation of metal ions to afford high storage capacity, whereas meso-/macropores improve metal ion transfer kinetics and electrolyte mobility (Zhang J. et al., 2020). A representative example of these strategies was provided by Zhang et al., who synthesized nitrogen-doped CSs (N-CSs) through a HTC method followed by treatment with NH3, in addition to the use of glucose as the carbon precursor and CTAB as a surfactant (Fig. 6(a)) (Zhang H. et al., 2019). The resulting material showed excellent lithium/sodium ion storage capacity which delivered reversible capacities of 578 and 281 mAh/g for LIBs and SIBs, respectively, at 20 mA/g. As shown in Fig. 6(b), when incorporated in LIBs, the N-CSs exhibited a capacity of 168.1 mAh/g at a high current density of 5 A/g, with a retention of 89.7 % even after 4,000 cycles. In contrast, the capacity of CSs (without nitrogen doping) was only 63.8 mAh/g, which was significantly lower than that of N-CSs. Similarly, in the case of trials with SIBs incorporating the N-CSs (Fig. 6(c)), a high capacity of 163 mAh/g was retained after 500 cycles, whereas the CSs showed a lower specific capacity of 52 mAh/g at 1 A/g. These results indicate that nitrogen doping can improve the conductivity of CSs and provide additional active sites, both of which are crucial for enhancing electrochemical performance.
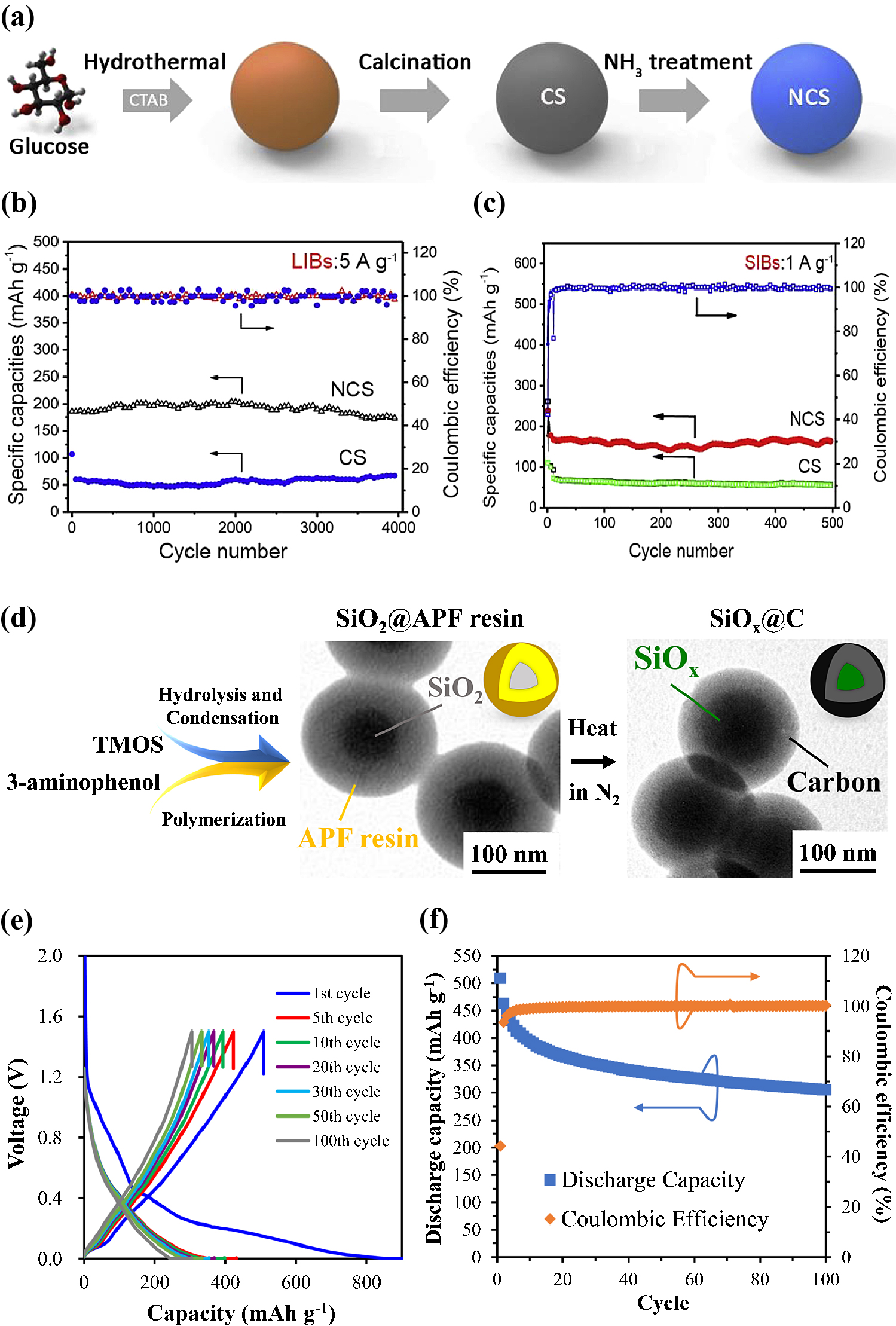
(a) An illustration showing the formation process of nitrogen-doped CSs (N-CSs), (b) cycling stability of N-CSs and CSs for LIBs at 5 A/g, and (c) cycling stability of N-CSs and CSs for SIBs at 1 A/g. Reprinted with permission from Ref. (H. Zhang et al., 2019). Copyright 2019, Elsevier. (d) An illustration showing the fabrication of SiOx@C core-shell particles, (e) galvanostatic charge-discharge profile, and (f) cyclic performance of SiOx@C particles as a LIB anode at different cycles. Reprinted with permission from Ref. (Cao et al., 2019). Copyright 2019, American Chemical Society.
Interestingly, certain non-carbon materials provide large theoretical capacities when used as battery anodes, including Si, Sn, Bi, P as well as various transition metal oxides (e.g., phosphides, sulfides, selenides) (Long et al., 2017). Even so, anodes made of these materials tend to undergo agglomeration and volume expansion/shrinkage during metalation/de-metalation and so exhibit poor cycling performance. CSs represent a means of addressing these problems and high-capacity carbon-based anode materials (e.g., SiOx/C, Fe3O4/C) have been intensively investigated in attempts to achieve high energy densities. Cao et al. reported the synthesis of carbon-coated SiOx (SiOx@C) core-shell particles through a sol-gel process (Cao et al., 2019). This work was based on the simultaneous hydrolysis-condensation of tetramethyl orthosilicate (TMOS) together with the polymerization of 3-aminophenol and formaldehyde in the presence of ammonia as a base catalyst and CTAB as a cationic surfactant, followed by the carbonization process (Fig. 6(d)). The resulting SiOx@C particles were investigated as an anode material for LIBs and exhibited a reversible capacity of 509 mAh/g at 100 mA/g with approximately 80 % capacity retention after 100 cycles (Fig. 6(e–f)). This significantly improved electrochemical performance can be attributed to the structure of the material. The core-shell structure of these particles ensured optimal contact with the carbon matrix, while the round shape of carbon shell was highly resistant toward stress. These factors improved the conductivity of SiOx and exerting the function of carbon.
On the cathode side, porous CSs have been successfully used as hosts for conductive sulfur in lithium-sulfur batteries (LSBs). The highly porous structure of these materials can accommodate a high proportion of sulfur while alleviating its isolating nature, thus facilitating the transport of electrons. Most studies show that sulfur is stored preferentially in the pores of carbon shells rather than in the interior of CSs, which is not conducive to inhibiting the shuttle effect and attaining high energy density. The process of sulfur material passing through the porous shell is difficult due to the capillary condensation effect and the absence of strong adsorption potential inside the carbon shell. As a result, the rational design of well-defined interior structure of CSs is an efficient method to suppress the “shuttle effect” of lithium polysulfides (LiPSs). Xu et al. proposed the fabrication of mesoporous nitrogen-doped yolk-shell CSs (N-YS-CSs) as novel sulfur encapsulators for LSBs through a Stöber method (Xu et al., 2018). The applied resorcinol-formaldehyde was acted as a carbon precursor and CTAB as a template to form the interior yolk and exterior shell under different hydrolysis polymerization rate. The N-YS-CSs@S cathode exhibited a high reversible capacity of 909 mAh/g at 0.2 C even after 500 cycles, which was higher than core-shell type architectures (323 mAh/g). The yolk-shell structure can effectively fulfill the synergistic effect of physisorption and chemisorption for LiPSs, and comprehensively absorb the LiPSs within the pores. In addition, the inner void between the yolk and shell provides sufficient cavity to accommodate the volume expansion during cycling, which contributes to the stability of the cycling performance. This strategy provides new ideas for the development of cathode structures for practical applications in LSBs. The structures, synthesis processes and electrochemical performance characteristics of CSs and active material/ CS composites used as electrode materials in rechargeable batteries are summarized in Table 4.
Structures, synthesis processes, and electrochemical performance characteristics of CSs and active material/CS composites intended for use as electrode materials in rechargeable batteries.
| No. | Material | Process | Template | Precursor | Battery | Reversible capability | Electrolyte | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | N-ACSs | HTC + Activation | — | Glucose | LSBs | 1003.7 mAh/g at 0.1 C | 1 M LiTFSI and 0.1 M LiNO3 in DOL/DME | (Xiang et al., 2018) |
| 2 | N-CSs | Surfactant-assisted HTC | — | Glucose | LIBs | 578 mAh/g at 20 mA/g | 1 M LiPF6 in DMC/EC/EMC | (Zhang H. et al., 2019) |
| SIBs | 281 mAh/g at 20 mA/g | 1 M NaPF6 in DMC/EC | ||||||
| 3 | PCSs | HTC + Activation | — | Starch | SIBs | 210.3 mAh/g at 50 mA/g | 1 M NaClO4 in DEC/EC | (Zhang J. et al., 2020) |
| 4 | CSs | Extended the Stöber method | — | Resorcinol and formaldehyde | PIBs | 241 mAh/g at 25 mA/g | 0.8 M KPF6 in DEC/EC | (Wang S. et al., 2020) |
| N-CSs | 3-Aminophenol and formaldehyde | |||||||
| 5 | MCSs | Spray drying + Carbonization | — | Chitosan | LSBs | 1163 mAh/g at 0.2 C | 1 M LiTFSI in DOL/DME | (Zhou H. et al., 2018) |
| 6 | N-MCSs | Soft template | PS-b-PAA | Dopamine | SIBs | 251 mAh/g at 100 mA/g | 1 M NaClO4 in PC + 5 % FEC | (Mao et al., 2020) |
| 7 | N-MCSs | Soft template | Pluronic F127 | Melamine and formaldehyde | SIBs | 373 mAh/g at 60 mA/g | 1 M NaPF6 in DEC/EC | (Guo et al., 2021) |
| 8 | YS-CSs | Soft template (Stöber method) | CTAB | Resorcinol and formaldehyde | PIBs | 314 mAh/g at 50 mA/g | 0.8 M KPF6 in DEC/EC | (Zhang H. et al., 2018) |
| 9 | N,O-YS-CSs | Soft template + HTC | CTAB | Resorcinol and formaldehyde | PIBs | 473.7 mAh/g at 20 mA/g | KPF6 in DEC/EC/PC | (Chong et al., 2021) |
| 10 | UHCSs | Hard template | SiO2 sphere | PTCDA | LSBs | 1305 mAh/g at 0.2 C | 1 M LiTFSI and 0.1 M LiNO3 in DOL/DME | (Song J. et al., 2018) |
| 11 | N,P-HPCSs | Hard template | SiO2 sphere | Dopamine | SIBs | 302 mAh/g at 100 mA/g | 1 M NaClO4 in DEC/EC + 5 % FEC | (Wang H. et al., 2020) |
| 12 | N-HMCSs | Hard template | SiO2 sphere | Dopamine | LSBs | 1656 mAh/g at 0.2 C | 1 M LiTFSI and 0.1 M LiNO3 in DOL/DME | (Zhang et al., 2015) |
| 13 | S,N-HCSs | Hard template | PMMA sphere | Resorcinol and formaldehyde | SIBs | 185 mAh/g at 500 mA/g | 1 M NaClO4 in DEC/EC + 2 % FEC | (Ye et al., 2016) |
| 14 | S,N-MHCSs | Hard template | CaCO3 sphere | Dopamine | SIBs | 240 mAh/g at 500 mA/g | 1 M NaClO4 in DEC/EC + 2 % FEC | (Ni et al., 2019) |
| 15 | SiOx@C core-shell | Soft template | CTAB | 3-Aminophenol and formaldehyde | LIBs | 509.2 mAh/g at 100 mA/g | 1 M LiPF6 in DMC/EC/EMC | (Cao et al., 2019) |
| 16 | Fe3O4@N-PCSs | Soft template | PS-b-PAA | Dopamine | LIBs | 1240 mAh/g at 100 mA/g | 1 M LiPF6 in DEC/EC | (Mao et al., 2019) |
| 17 | Se@N-MCSs | Soft template | Pluronic F127 | Dopamine | SIBs | 378 mAh/g at 100 mA/g | 1 M NaClO4 in DEC/EC + 5 % FEC | (Sun et al., 2021) |
| 18 | Sn4P3-C core-shell | HTC + Phosphorization | — | Glucose and potassium stannate | LIBs | 700 mAh/g at 500 mA/g | 1 M LiPF6 in DEC/EC + 2 % VC | (Choi et al., 2018) |
| SIBs | 680 mAh/g at 500 mA/g | 1 M NaClO4 in DMC/EC + 5 % FEC | ||||||
| 19 | Bi@C nanospheres | HTC | — | Glucose and bismuth nitrate | SIBs | 400.3 mAh/g at 200 mA/g | 1 M NaPF6 in DME | (Zhu et al., 2020) |
| 20 | SnS QDs@N-CSs | HTC | — | Dopamine and tin(II) chloride | SIBs | 280 mAh/g at 100 mA/g | 1 M NaClO4 in EC/PC + 10 % FEC | (Veerasubramani et al., 2020) |
Typically, porous CSs have been employed as electrode materials in commercial electrical double-layer capacitor (EDLC) devices (Zhang M. et al., 2018). The spherical structure of these materials can decrease the distance that electrolyte ions are required to migrate so that an electric double-layer easily forms between the electrode and electrolyte. The capacitance of EDLCs is directly related to the contact area of the electrode with the electrolyte. In principle, increasing the pore volume, SSA and electrical conductivity of porous CS electrode will enhance charge accumulation in the electrical double layer formation and so improve capacitance (Pang et al., 2018b; Tang et al., 2018). It is generally considered that the presence of micropores in carbon materials significantly increases the SSA, which in turn plays an important role in enhancing specific capacitance. However, some investigations have discovered that ultrahigh SSAs may result in decreased volumetric capacitance as a consequence of relatively low packing densities (Kitamoto et al., 2022; Pang et al., 2018a). For this reason, it is critical to adjust pore sizes to maximize the ion-accessible SSA while decreasing dead volume. Both theoretical and experimental studies have confirmed that the highest capacitance is obtained in the case that the pore size (primarily micropores) is well matched to the ion size of the liquid electrolyte, because this scenario maximizes charge accommodation. However, the slow ion diffusion and desolvation in sub-nanometer micropores mean that high capacitance can only be realized at low or moderate charge-discharge rates, which limits the power density and rate capability. This issue can be resolved by incorporating macro-/mesopores into microporous carbon structures. In such cases, the mesopores can act as reservoirs to shorten ion transport pathways and so boost ion diffusion, allowing for high capacitance retention during rapid charging/discharging (Wang et al., 2018; Zhang et al., 2017). Thus, optimizing the proportions of micro-, meso-, and macropores in a porous CS electrode is critical to attaining high specific capacitance and rate performance.
The incorporation of heteroatoms (e.g., N, O, S, B, P) into porous CSs also plays a vital role to improve the electrochemical performance. Several studies have been conducted on the use of porous CSs doped with a variety of heteroatoms such as nitrogen (Xiong et al., 2017; Zhang L. et al., 2019), sulfur (Liu et al., 2017), or nitrogen co-doped with sulfur (Lv et al., 2020), oxygen (Chen et al., 2017; Liu S. et al., 2016), and phosphorus (Zhang et al., 2017) as electrodes in supercapacitors. Such modifications provide additional active sites for reversible redox reactions and so raise the overall capacitance of the sample. Yan et al. fabricated N,P,S-codoped hierarchically porous CSs (N,P,S-PCSs) by utilizing silica colloid as a hard template, polyaniline as both a carbon and nitrogen precursor, phytic acid as a phosphorus precursor, and ammonium persulfate as a sulfur precursor (Yan et al., 2018). The well-dispersed pores provide a facile ion transportation path within the electrode. The resulting N,P,S-PCSs exhibited both high gravimetric and volumetric specific capacitance values of 274 F/g and 219 F/cm3 at 0.5 A/g, which revealed a superior electrochemical performance. After 10,000 cycles, the N,P,S-PCSs samples showed the long-term cycling stability with 95 % initial capacitance retention at 10 A/g. This is mainly attributed to the uniform doping of N, P, and S as well as the hierarchically porous structure of the N,P,S-PCSs. Table 5 summarizes the supercapacitor performance and the main characteristics of porous CSs prepared by various synthetic processes.
Structures, synthesis processes, and electrochemical performance characteristics of porous CSs as electrode materials in supercapacitors.
| No. | Material | Process | Template | Precursor | Specific capacitance | Electrolyte | Reference |
|---|---|---|---|---|---|---|---|
| 1 | ACSs | HTC + Activation | — | Xylose | 238.1 F/g at 1 A/g | 1 M Na2SO4 | (Sun et al., 2020) |
| 2 | PCSs | HTC + Activation | — | Starch | 369.8 F/g at 1 A/g | 1 M H2SO4 | (Zhang J. et al., 2020) |
| 331.4 F/g at 0.5 A/g | 6 M KOH | ||||||
| 3 | PCSs | HTC + Activation | — | GAML | 303.7 F/g at 0.04 A/g | 6 M KOH | (Hao et al., 2018) |
| 4 | N-HCSs | Extended the Stöber method | — | Resorcinol and formaldehyde | 196 F/g at 1 A/g | 6 M KOH | (Zhang L. et al., 2019) |
| N-YS-CSs | 242 F/g at 1 A/g | ||||||
| N-SCSs | 160 F/g at 1 A/g | ||||||
| 5 | Pomegranate-like CSs | Spray drying + Activation | — | 3-Aminophenol and formaldehyde | 338 F/g at 1 A/g | 6 M KOH | (Feng et al., 2019) |
| 6 | HCSs | Spray drying + Carbonization | — | Kraft lignin | 31.8 F/g at 0.2 A/g | 6 M KOH | (Cao et al., 2021a) |
| 7 | PCSs | Spray drying + Carbonization | — | Kraft lignin | 66.5 F/g at 10 mV/s | 6 M KOH | (Kitamoto et al., 2022) |
| 8 | HPCSs | Spray drying + Carbonization | — | Sodium lignosulfonate | 284 F/g at 0.1 A/g | 7 M KOH | (Pang et al., 2018b) |
| 9 | N,S-PCSs | Spray pyrolysis | — | Coal | 308 F/g at 1 A/g | 6 M KOH | (Lv et al., 2020) |
| 10 | PCSs | Spray pyrolysis + Activation | — | Glucose | 240 F/g at 0.5 A/g | 6 M KOH | (Tang et al., 2018) |
| 80 F/g at 0.5 A/g | 1 M TEABF4/AN | ||||||
| 11 | N-HCSs | Spray pyrolysis + Carbonization | — | Glucose and glucosamine | 266 F/g at 0.2 A/g | 1 M H2SO4 | (Qu et al., 2018) |
| 12 | N-MCSs | Soft template + Activation | PS-b-PEO | Dopamine | 170 F/g at 1 A/g | EMIMBF4 | (Tang et al., 2017) |
| 111 F/g at 1 A/g | 1 M TEABF4/AN | ||||||
| 13 | N-PCSs | Soft template + Activation | Pluronic F127 | Dopamine | 433 F/g at 0.5 A/g | 6 M KOH | (Xiong et al., 2017) |
| 14 | PCSs | Soft template + Spray pyrolysis | Pluronic F127 | Larch sawdust (biomass) | 338.8 F/g at 0.2 A/g | 6 M KOH | (Song et al., 2019) |
| 15 | N,O-CSs | Soft template + HTC | Pluronic F127 | 3-Aminophenol and formaldehyde | 309 F/g at 0.1 A/g | 6 M KOH | (Liu S. et al., 2016) |
| 16 | N-PCSs | Soft template + HTC | Pluronic F108 | Phenol and formaldehyde | 365 F/g at 0.5 A/g | 6 M KOH | (Liang et al., 2019) |
| 17 | N-HMCSs | Soft template (Extended the Stöber method) | CTAC | Resorcinol and formaldehyde | 300 F/g at 1 A/g | 1 M H2SO4 | (Liu C. et al., 2016) |
| N-YS-MCSs | 178 F/g at 1 A/g | ||||||
| 18 | HCSs | Soft template | CTAB | Phenol and formaldehyde | 265 F/g at 0.5 A/g | 6 M KOH | (Du et al., 2020) |
| 19 | APCSs | Soft template | DDAB | 3-Aminophenol and formaldehyde | 256 F/g at 0.2 A/g | 6 M KOH | (Li et al., 2021) |
| 20 | N-PCSs | Soft template + HTC | [C12mim]Br | 3-Aminophenol and amphiphilic aliphatic aldehydes | 181 F/g at 1 A/g | 6 M KOH | (Jia et al., 2022) |
| 21 | N-MCHSs | Soft template + HTC | Gemini | 3-Aminophenol and formaldehyde | 240 F/g at 0.2 A/g | 6 M KOH | (Li et al., 2020) |
| 22 | N-HPCSs | Hard template + Activation | SiO2 sphere | Dopamine | 353 F/g at 0.5 A/g | 6 M KOH | (Song Z. et al., 2018) |
| 23 | N,P-PCSs | Hard template | SiO2 sphere | Melamine and formaldehyde | 208 F/g at 0.5 A/g | 6 M KOH | (Zhang et al., 2017) |
| 24 | N-HCSs | Hard template | SiO2 sphere | m-Phenylenediamine and formaldehyde | 234 F/g at 0.5 A/g | EMIBF4 | (Xu et al., 2021) |
| 221 F/g at 0.5 A/g | TEABF4/AN | ||||||
| 25 | N,P,S-PCSs | Hard template | SiO2 sphere | Aniline | 274 F/g at 0.5 A/g | 6 M KOH | (Yan et al., 2018) |
| 26 | N,O-HCSs | Hard template + Activation | PS sphere | Pyrrole | 535 F/g at 0.2 A/g | 6 M KOH | (Chen et al., 2017) |
| 473 F/g at 0.2 A/g | 1 M H2SO4 | ||||||
| 27 | N-PCSs | Hard template | CaCO3 sphere | Dopamine | 270 F/g at 0.5 A/g | 6 M KOH | (Guo et al., 2018) |
| 28 | N-HCSs | Hard template | Cu2O sphere | 3-Aminophenol and formaldehyde | 263.6 F/g at 0.5 A/g | 6 M KOH | (Zhang D. et al., 2020) |
Experimentation with spherical particles having attractive structures and multiple functions has proceeded for many years and these materials are expected to play a significant role in future energy sources. This review provides an overview of the latest advances and recent progress in the synthesis of CSs, with a particular focus on the applications of these materials in electrochemical energy storage. There have been significant advances in the fabrication of CSs with well-designed, controllable morphologies, desirable textural properties and adjustable functionalities. The primary techniques for fabricating CSs comprise template-free, soft template and hard template approaches, all of which are discussed in detail herein to provide a clear understanding of the capability of each synthesis route in terms of controlling structural features. It is vital to obtain specific chemical compositions and morphologies when fabricating CSs with targeted functionalities related to potential applications in rechargeable batteries and supercapacitors. This review demonstrates that CSs have been extensively employed because the porosity and other structural parameters of these materials are readily tuned. As such, CSs are of particular interest with regard to addressing critical issues related to rate capability and cycling stability, as well as facilitating their application in emerging energy storage devices. Despite the obvious advantages and significant achievements summarized above, CSs still face numerous challenges that need to be addressed before practical applications in energy storage devices are feasible. A number of suggested future research directions related to the advancement of this field of study are detailed below.
(1) The electrochemical performance of CSs requires further improvement to allow practical usage. As an example, the spherical carbon-based materials having core-shell structures with porous shells are especially appealing as anode materials because they are able to host a number of active species that provide the electrical conductivity required for stable cycling and efficient use. It is important to prepare CS materials with pore structures that are tunable within the micropore and mesopore size ranges and to construct hierarchical structures by connecting micropores to mesopores and macropores. These features would improve the transport of ions and provide abundant active sites to increase the ion accessible SSA, leading to enhance the performance of energy storage devices. It will also be important to consider the packing density of CSs because this is a critical parameter that is rarely investigated. It is worth noting that the packing density of CSs is generally low (< 0.5 g/cm3). CSs typically possess hollow and porous structures, which result in improving the mass-ratio of the powder and energy density but decrease the volumetric energy density of the device. Consequently, some important applications of CSs are limited.
(2) The large-scale industrial applications of CSs are still restricted by the cost and the scale of production, which is vastly different from the estimated and makes them delayed in their adoption. For instance, to enhance the SSA and porosity, highly corrosive chemical activation treatment (e.g., KOH, H3PO4) followed by the removal of impurities is required. These processes typically consume extra energy and produce significant waste streams. Therefore, green chemical engineering processes are essential to the sustainable fabrication of porous CSs for energy storage devices. Future research may focus on the fabrication of CSs using less toxic and greener chemical activating agents at a reasonable cost.
Furthermore, it is important to note that the selection of carbon precursor has a pronounced effect on the performance of CSs. Biomass-based porous CSs are a promising material for emerging applications. However, biomass usually contains pollutants and other impurities. Important questions remain unanswered concerning the impacts of using these materials to construct CSs. How will these impurities migrate and transform during the preparation and application of CSs? What is the effect of these contaminants on the properties and performance of CSs? Answers to these questions must be determined to advance the use of biomass as a precursor for CS materials.
(3) The development of reliable synthetic pathways is still an emerging requirement and may be required to allow the scalable production of CSs with control over their structural properties. The innovation and simplicity of the new green synthesis protocol will be a prerequisite to produce CSs in conjunction with reduced costs, minimal environmental impact, and improved quality to facilitate their usage in large-scale energy storage devices. The use of green activation strategies typically provides enhanced yields. However, these approaches have been underdeveloped and more detailed studies are needed for their optimization and advancement.
(4) Heteroatom doping is generally used to modify the surface chemistry of carbon materials but overdoping can vitiate the porous structure and an excess of some atoms can impair the electrical conductivity of the carbon. The future development of porous CSs will require an in-depth understanding of the structure-function relationship to allow the formation of desired structure and surface chemistry that meet the demands associated with various applications.
(5) The rapid evolution of CSs has been associated with advances in energy storage technologies. However, there remains an incomplete understanding of the relationships between structure and the electrochemical properties of CSs. More advanced characterizations in conjunction with numerical simulations may provide better insight into the connection between structures and electrochemical properties or reaction mechanisms.
In the future, there is likely to be increasing interest in CS materials for specific scientific and industrial applications and these materials will be used more frequently to support the development of energy storage devices. A better understanding of structure-performance relationships is also anticipated. Moreover, greener and simpler synthesis methods with reduced environmental impacts will be developed to enable the full use of renewable resources. There is no doubt that many opportunities are hidden, and the researchers should investigate more on utilizing the tremendous potential of functionalized CSs in the applications discussed herein. The progress achieved to date is expected to inspire future research and the authors hope that this review paper will accelerate further work toward a future sustainable society.
This work was supported by a JSPS KAKENHI Grant Number 19H02500, International Network on Polyoxometalate Science at Hiroshima University, the JSPS Core-to-Core Program, the Hosokawa Powder Technology Foundation, and the Information Center of Particle Technology, Japan. We thank Michael D. Judge, MSc, from Edanz Group for editing a draft of this manuscript.
The video data of hollow carbon particle formation prepared via ultrasonic spray pyrolysis using phenolic resin (−) and PSL (+) is available publicly in J-STAGE Data (https://doi.org/10.50931/data.kona.21014023).
The video data of porous carbon particle formation prepared via ultrasonic spray pyrolysis using phenolic resin (−) and PSL (−) is available publicly in J-STAGE Data (https://doi.org/10.50931/data.kona.21014023).
Kiet Le Anh Cao
Kiet Le Anh Cao received his B.Sc. from University of Science, Vietnam National University, Ho Chi Minh City in 2014 and M.Sc. from Hanyang University, Korea in 2018. He obtained his Ph.D. degree in Chemical Engineering from Hiroshima University in 2021. He is currently working as a post-doctoral researcher in the Department of Advanced Science and Engineering, Hiroshima University, Japan. His research focuses on the rational design and development of novel nanostructured materials for energy storage and conversion.
Ferry Iskandar
Ferry Iskandar studied transport phenomena at Hiroshima University, Japan, where he earned his doctorate. From 2007 until 2010, he worked as a faculty staff of Hiroshima University’s Department of Chemical Engineering. He is currently an Associate Professor at the Department of Physics at the Institut Teknologi Bandung in Indonesia. Since 2016, Ferry Iskandar has also been a researcher at the Indonesian Research Center for Nanosciences and Nanotechnology. His broad research interests include material synthesis and physical characterisation, with an emphasis on nanostructured materials and their applications in the fields of photoenergy conversion and energy storage, including batteries and capacitors.
Eishi Tanabe
Eishi Tanabe is a senior researcher of Hiroshima Prefectural Technology Research Institute. He received his bachelor’s degree and master’s degree of science in physics from Hiroshima University in 1993 and 1995, respectively. He started his career with electron microscopy in Hiroshima Prefecture Industrial Research Center in 1995. He received his Ph.D. degree in material science from Shimane University in 2005. His current research interests are TEM and FIB-SEM Tomography.
Takashi Ogi
Takashi Ogi is a Full Professor in the Department of Advanced Science and Engineering, Hiroshima University. He received his Ph.D. degree in Chemical Engineering in 2008 from Hiroshima University. He was an Assistant Professor at Osaka Prefecture University from 2008–2010 and Associate Professor at Hiroshima University from 2015–2021. His current research interests include synthesis of nanostructured particles materials via liquid phase and aerosol process. Especially, he focuses on the development of rare earth free/less nanomaterials, and recovery of rare metal using biosorption.