2021 Volume 10 Issue 1 Pages A0100
2021 Volume 10 Issue 1 Pages A0100
CO3−• and O2−• are known to be strong oxidizing reagents in biological systems. CO3−• in particular can cause serious damage to DNA and proteins by H• abstraction reactions. However, H• abstraction of CO3−• in the gas phase has not yet been reported. In this work we report on gas-phase ion/molecule reactions of CO3−• and O2−• with various molecules. CO3−• was generated by the corona discharge of an O2 reagent gas using a cylindrical tube ion source. O2−• was generated by the application of a 15 kHz high frequency voltage to a sharp needle in ambient air at the threshold voltage for the appearance of an ion signal. In the reactions of CO3−•, a decrease in signal intensities of CO3−• accompanied by the simultaneous increase of that of HCO3− was observed when organic compounds with H–C bond energies lower than ∼100 kcal mol−1 such as n-hexane, cyclohexane, methanol, ethanol, 1-propanol, 2-propanol, and toluene were introduced into the ion source. This clearly indicates the occurrence of H• abstraction. O2−• abstracts H+ from acid molecules such as formic, acetic, trifluoroacetic, nitric and amino acids. Gas-phase CO3−• may play a role as a strong oxidizing reagent as it does in the condensed phase. The major discharge product CO3−• in addition to O2−•, O3, and NOx• that are formed in ambient air may cause damage to biological systems.
Basic data on gas-phase and condensed-phase reactions of CO3−• and O2−• are of importance in aeronomy, environmental chemistry, biology, and medicine. In Scheme 1, the mechanisms for the formation of CO3−•, HCO3−, and NO3− via consecutive gas-phase ion/molecule reactions originating from O2, H2O, and N2 are summarized. In gas phase reactions of CO3−•, O−• transfer reactions with the formation of CO2 were found to be the major reaction channels for NO, NO2, SO2,1) N2O5,2) and 2,4,6-trinitrotoluene.3) Fehsenfeld et al.4) and van der Linde et al.5) reported that CO3−• reacted with HNO3 via a proton transfer reaction to form NO3− and HCO3•. In addition, van der Linde et al.6) studied gas-phase reactions of CO3−• with formic acid (HCOOH) to form [HCOO−····OH•] using FT-ICR mass spectrometry. Ninomiya et al.3) predicted that CO3−• reacts with H2O2 to form the cluster ion, O2−•····H2CO3. As of this writing, however, no H• abstraction by CO3−• in the gas phase has been reported, even though H• abstraction reactions are a major concern due to its potential for causing damage in biological systems.7) Thus, it would be of interest to examine the issue of whether CO3−• also abstracts H• from organic compounds in the gas-phase. Kawashima et al. measured product ions formed from collisionally excited cluster ions of [CO3−•+M] in the gas phase with M being 16 amino acids and organic acids.8) They detected HCO3− as the major product ion formed from amino acids. This indicates the occurrence of H• abstraction by CO3−• in collisionally excited cluster ions of [CO3−•+M]*. Their results may give some insight into the subject of H• abstraction reactions of CO3−• in the gas phase.

In reactions of CO3−• in aqueous solutions, Elango et al.9) reported that CO3−• reacted with aliphatic amines by (i) a H• abstraction to form HCO3− and (ii) an electron transfer to form CO32− (one electron oxidation). The former is more probable in cases of primary amines, while tertiary amines reacted via electron transfer. Clifton and Huie10) measured rate constants in aqueous solutions for H• abstraction reactions of CO3−• with several saturated alcohols and cyclic ethers. The Arrhenius pre-exponential factors ranged from 2×108 to 1×109 M−1s−1 and the activation energies ranged from 3.8 to 6.9 kcal mol−1 (1 cal=4.18 J). Crean et al.11) investigated the oxidation of single-stranded oligonucleotides by CO3−•, leading to the generation of intrastranded cross-links between guanine and thymine bases that were separated by cytosines. Roginskaya et al.12) studied the efficacy and site specificity of H• abstraction from DNA 2-deoxyribose by CO3−• and also evaluated the selectivity of damage in double-stranded DNA. Karmakar and Datta13) reported on the reactivity of CO3−• for six amino acid chains (Cys, Met, Phe, Tyr, His and Trp) using state-of-the-art density functional theory. They reported that CO3−• causes oxidative damage to amino acid residues predominantly via H• abstraction with moderate to high rate constants.
Various types of gas-phase ion/molecule reactions of O2−• have also been investigated, including [1] SN2 (nucleophilic second-order substitution) reactions for CH3Br,14) CH3Cl,14) CF3CO2CH3,14) and CH3CO2CH3,14) [2] charge (electron) transfer reactions for CCl2F2,15) CCl3F,15) SF6,16–18) 2,4,6-trinitrotoluene,19) and O3,20) [3] H+ abstraction reactions for CF3SO3H,21) HCl,21,22) FSO3H,23) and HNO3,4) and [4] clustering reactions for CH3CN,23) (CF3)2CO,14) H2C=CHCN,14) (CH3)2CO,14) and higher hydrocarbons.24)
In a physiological environment, the superoxide anion O2−• can function as an oxidant or a reductant, and the dismutation reaction, 2O2−•+2H+ → H2O2+O2, is an example of this.7) The reaction (1) of O2−• with NO• has received special attention due to the fact that peroxynitrite, ONOO−, a strong biological oxidant, is generated.
 | (1) |
Peroxynitrite reacts with CO2 to produce CO3−• via reactions (2) and (3).
 | (2) |
 | (3) |
That is, O2−• triggers the formation of CO3−•, which is a highly oxidative species in biological systems.
In the present study, the gas-phase reactions of CO3−• and O2−• with various organic molecules such as hydrocarbons, alcohols, and acids were investigated. It was observed that CO3−• abstracts H• from methanol, ethanol, 1-propanol, 2-propanol, n-hexane, cyclohexane, and toluene, to form HCO3−. In the reaction of CO3−• with H2O2, O2−•····H2CO3 cluster ions were detected. In contrast, the only type of reaction for O2−• observed in this experiment was H+ abstraction reactions from various acid molecules (i.e., formic acid, acetic acid, nitric acid, trifluoroacetic acid, and amino acids).
Reagent grade n-hexane, cyclohexane, benzene, toluene, methanol, ethanol, 1-propanol, 2-propanol, acetone, acetonitrile, formic acid, acetic acid, trifluoroacetic acid, nitric acid, and amino acids (leucine, isoleucine, alanine, and phenylalanine) were purchased from FUJIFILM Wako Pure Chemical (Osaka, Japan). CO2 gas (liquefied CO2, 99.9%, Nitto-Bussan Corp., Yamanashi, Japan) and O2 gas (ZERO-U, >99.999%, Sumitomo Seika Chemicals Corp., Tokyo, Japan) were used as reagent gases.
Ion source assembly for the formation of CO3−•The mass spectrometric measurements were performed with a time-of-flight mass spectrometer (AccuTOF, JEOL, Akishima, Tokyo, Japan). Figure 1(a) shows the assembly of the cylindrical ion source tube that was used for the formation of CO3−•. The ambient air open distance between the terminal end of the cylindrical tube and the ion sampling orifice of the mass spectrometer was 8 mm. When CO2 was used as the reagent gas for the formation of CO3−•, O2−• with a relative intensity of about 20–30% compared to CO3−• was unavoidable, as shown in Fig. S1. The formation of O2−• may originate from O2 contamination in the CO2 reagent gas or O2 formed by the decomposition of the CO2 reagent gas in the corona discharge plasma. In contrast, when O2 was used instead of CO2 as the reagent gas, CO3−• was generated as the major ion and the formation of O2−• was negligible (see Fig. 2(a)). This indicates that O3−• produced by the DC discharge of the reagent O2 gas was efficiently converted into CO3−•. The source of the carbon for the formation of CO3−• may be the impurity of CO2 in the O2 reagent gas, adsorbed CO2 and/or CO on the wall of the gas pipe line.

The use of a stainless steel throttle shown in Fig. 1(a) is essential for the generation of CO3−• as the major ion. When the throttle was not used, the back diffusion of air into the inside of the tube could not be avoided, even when the tip of the needle electrode was recessed a distance of 10 mm from the exit of the glass tube. The back diffusion of air was readily recognized by the detection of HCO3− originating from the moisture in the air (see Scheme 1(b)). When the tip of the needle electrode was recessed a distance of 30 mm from the exit of the mesh-covered glass tube with the throttle inserted near the exit, the back diffusion of air was nearly completely avoided and CO3−• ions were formed as the only major reactant ion flowing out of the glass tube. Thus, the back diffusion of reactant vapor introduced into the plasma region of the ion source appears to be negligible in the present experimental setup.
The O2 reagent gas with a flow rate of 3 L min−1 was ionized by a direct current (DC) corona discharge. The consecutive reactions (4)–(11) took place in ambient air (Scheme 1(a)). The major ion O2−• that was generated was formed by an electron attachment reaction (4), then reacted with O3 (major neural product in corona discharge of O2 reagent gas) to form O3−• by the charge transfer reaction (10). O3−• further reacted with the CO2 impurity in the O2 carrier gas to form CO3−• (reaction (11)).
 | (4) |
 | (5) |
 | (6) |
 | (7) |
 | (8) |
 | (9) |
 | (10) |
 | (11) |
The changes in the enthalpy for reactions (10) and (11) were calculated to be −37.619) and −11.825) kcal mol−1, respectively, and reactions (10) and (11) proceed with nearly collision rates.23)
O2−• formation using a sharp metal needle in ambient airThe simple experimental setup for this process is shown in Fig. 1(b). The formation of O2−• as the major ion was only observed when a high-frequency (15 kHz) threshold voltage for the appearance of the ion signal was applied to the needle in ambient air. This phenomenon is attributed to the field electron emission from the tip of the sharp needle (see the latter section).
By using the ion source shown in Fig. 1(a), reactions of CO3−• with hydrocarbons (n-hexane, cyclohexane, benzene, and toluene), alcohols (methanol, ethanol, 1-propanol, and 2-propanol), acetonitrile, acetone, water, and H2O2 were examined. A 10 μL aliquot of a liquid sample was placed on the well of the heater shown in Fig. 1. The heater temperature was maintained at a temperature of about 30°C above the boiling point of the liquid.
As an example, experimental results obtained for n-hexane are shown in Fig. 2. Figure 2(a) shows the mass spectrum before sample introduction, in which the only detected ion was CO3−•. Figures 2(b) and 2(c) show the mass spectra that were obtained when 10 μL liquid hexane was placed on the heater twice at 0.19 min and at 0.42 min. On the introduction of the sample, HCO3− (m/z 61) appeared at 0.19 min and at 0.42 min, respectively. Figures 2(d) and 2(e) show the extracted ion current (EIC) chronograms for CO3−• and HCO3−, respectively. A sharp decrease in CO3−• and an increase in HCO3− were simultaneously observed when the sample was introduced into the ion source. These results clearly indicate that CO3−• abstracts H• from n-C6H14, with the formation of HCO3− in the gas phase (reaction (12)).
 | (12) |
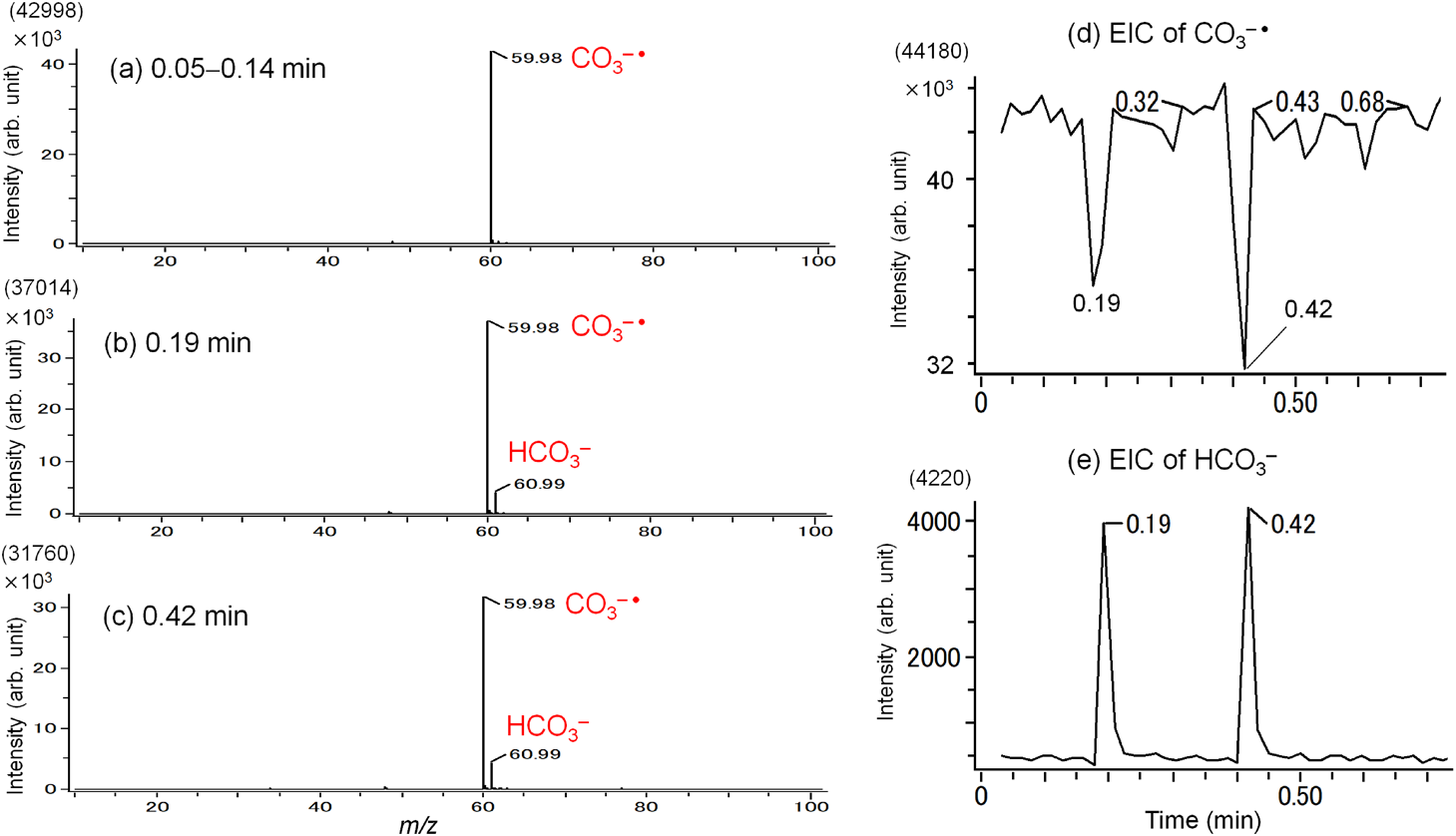
Gas-phase H• abstraction reactions by CO3−• have not been extensively explored, which may be due to the fact that reactant molecules are not detected as ions in H• abstraction reactions. In this work, H• abstraction reactions were also detected for cyclohexane, toluene, methanol, ethanol, 1-propanol, and 2-propanol (data not shown). However, H2O, benzene, acetone, and acetonitrile did not show any noticeable reactivity toward CO3−• under the present experimental conditions.
From the value for the heat of formation of HCO3− (−173.9 kcal mol−1),26) the bond energy of H•····CO3− was estimated to be 105.7 kcal mol−1. Table S1 summarizes the H–C bond energies of hydrocarbons and alcohols and that of H–O bond of H2O.25,26) Roughly speaking, CO3−• abstracts H• from molecules that have H–C bond energies equal to or smaller than ∼100 kcal mol−1. In one exceptional case, the ion intensity of CO3−• did not change and HCO3− was not detected when acetonitrile (bond energy of H–C bond of methyl group: 93.2 kcal mol−1) was introduced into the ion source. At present, we have no explanation for why CO3−• did not show any noticeable reactivity toward acetonitrile, but there may be a substantial entropy barrier for this reaction. In the present work, occurrence/non-occurrence experiments on the reactivity of CO3−• in the gas phase were conducted for the first time, though no quantitative information such as rate constants was obtained.
In order to determine whether or not CO3−• can abstract a hydrogen atom from organic molecules (Ms), DFT calculations were carried out for the reaction of CO3−• with three representative molecules, namely, benzene, n-hexane and toluene. The computational method is M06-2X/6-311++G(2p,d), which gives very accurate thermochemical data and activation energies.27) Using this approach, the geometries of the transition state (TS) were determined. Subsequently, the intrinsic reaction coordinate (IRC) was traced to obtain the geometries of weakly bound complexes, CO3−•····M and HCO3−+[M−H]•. Gaussian 16 was used for all calculations.28) Fig. S2 exhibits the Braumann-type energy changes expressed by differences in electronic and zero-point vibrational energies. Fig. S3 shows TS geometries. In Fig. S2, the reaction for benzene has a large endoergicity, +14.60 kcal/mol, for the formation of HCO3−+[M−H]• and can be ruled out since it is in agreement with the experimental results. In contrast, for n-hexane and toluene, H• abstraction reactions are likely to occur. Spin densities were calculated for the “HCO3−····[M−H]•” complex, and it was confirmed that the reaction involves the abstraction of a hydrogen atom and does not produce HCO3•····[M−H]− (i.e., no proton transfer). The occurrence of H• abstraction reactions may be due to the fact that the energy barrier of the transition state is of the same order as the bond energies of the ion–molecule complexes. In addition, the tunneling effect of H• may contribute to these reactions, i.e., the wave matter of H• penetrates through the barrier without crossing the barrier.
In our previous work,3) based on theory, we predicted that CO3−• reacts with H2O2 to form O2−•····H2CO3 cluster ions. However, this cluster ion was not detected experimentally in our previous study.3) This may be due to the relatively low abundance of CO3−• generated by the ambient-air corona discharge. Figures 3(a) and 3(b) show the mass spectra before and after the introduction of H2O2 into the ion source shown in Fig. 1(a), respectively. In Fig. 3(b), the cluster ion O2−•····H2CO3 (m/z 94) in addition to the base peak for O2−• was clearly detected. The cluster ion O2−•····H2CO3 may be formed by the collisional stabilization of the intermediate complex, [O2−•····H2CO3]*.
 | (13-1) |
 | (13-2) |

The cluster ion of O2−•····H2CO3 detected in this work suggests that the unimolecular decomposition of H2CO3 to H2O and CO2 is frozen by the strong bond formation between O2−• and H2CO3. In our previous study,3) the binding energy of O2−•····trans-trans H2CO3 was calculated to be 58.40 kcal mol−1 using highly accurate theoretical calculations (G3MP2B3).3)
Field electron emission by the application of high-frequency voltage to the sharp metal needleAs shown in Scheme 1 in the ambient-air corona discharge, O2−• as the intermediate ion is rapidly converted into CO3−• as the terminal product ion via reactions (4)–(11). Thus, investigating the reactivity of O2−• as the single reactant ion is difficult using corona discharge as the ion source. In fact, previous investigations of the reactions of O2−• have primarily been conducted using flowing afterglow techniques.23) However, we discovered that O2−• was only formed as the product ion only when a high frequency voltage, but not a DC voltage, was applied to the needle electrode in the open ambient air (see Fig. 1(b)).
Figures 4(a)–4(d) show negative-mode mass spectra when a 15 kHz alternating current (AC) high voltage was applied to the needle. At the threshold voltage of ±1150 V for the detection of ion signals, O2−• was detected as the product ion. If gas breakdown occurred with this voltage, O3 must be formed by the decomposition of O2 in the plasma (see Scheme 1(a)). The total absence of O3−• (m/z 48) and CO3−• (m/z 60) indicated that gas breakdown followed by the generation of a corona discharge did not occur at this threshold voltage of ±1150 V. The formation of O2−• as the predominant ion suggests that free electrons were generated and they were converted into O2−• in ambient air by the electron attachment reaction (4). It is likely that free electrons were generated by the field electron emission from the tip of the needle electrode. The conceptual scenario for the tunneling electron emission is depicted in Fig. S4. As shown in Fig. 4(b), O3−• and CO3−• started to be detected when the AC voltage was increased to ±1250 V. This suggests that the AC corona discharge started to contribute to ion formation in addition to field electron emission at ±1250 V. With a further increase in the AC high voltage to ±1600 V, NOx− ions (x=2, 3) originating from the decomposition of N2 (in Scheme 1(c)) were detected.

Figures 4(e)–4(h) show negative-mode mass spectra when a negative DC high voltage was applied to the needle. Figure 4(e) shows the mass spectrum when a threshold voltage of −1500 V was applied to the needle for the observation of ion signals. It should also be noted that the threshold voltage of −1500 V is considerably higher (more negative) than that of the negative-phase AC voltage of −1150 V shown in Fig. 4(a). It is apparent that in the DC mode of operation, field electron emission is largely suppressed and a corona discharge is directly generated at −1500 V. Furthermore, in the DC mode, the field electron emission must occur at around −1150 V at the precise moment of when a high voltage is applied to the needle. However, due to the continuous application of a negative DC high voltage to the needle, free electrons were emitted and O2−• formed by electron attachment may have accumulated near the tip of the electrode. The accumulated negative charge near the tip of the needle should result in the formation of a space-charge field that shields the electric field at the tip of the needle. Due to the decrease in the electric field, the field strength at the needle tip becomes lower than that needed for the field electron emission. Such a build-up of the space charge field can be avoided by the application of an AC voltage because the free electrons and resulting O2−• that are produced in the negative voltage phase can be completely scavenged by the metal needle by the subsequent positive phase high voltage that is applied, as shown in Fig. 5.

A separate experiment was performed to examine the electron scavenging effect suggested above. A high voltage pulse of −3000 V with a pulse width in the range of 200 ns to 100 μs was applied to the needle with and without the application of a positive bias voltage of +500 V. The pulse repetition rate was 20 Hz. With a pulse width of 200 ns, the ion signals started to be detected at −3000 V. Figs. S5(a)–S5(d) show mass spectra obtained with a bias voltage of 0 V. Even with a pulse width of 200 ns, discharge product ions such as NO2− and CO3−• were detected. With no bias voltage, the electrons emitted by the tunneling effect are accelerated by the strong electric field resulting in the breakdown of the gas. In contrast, only O2−• was detected with a pulse width of 200 ns when a bias voltage of +500 V was applied, as shown in Fig. S5(e). It is evident that a bias voltage of +500 V is effective for scavenging electrons that are emitted by the tunneling effect and this suppresses the occurrence of corona discharge. However, a pulse width of 1 μs in Fig. S5(f) is sufficient for gas breakdown to occur as discharge product ions such as CO3−• start to be detected.
The scavenging effect of the build-up of negative charges near the needle tip in the positive phase of AC high voltage should be dependent on the frequency of the AC high voltage. Fig. S6 shows mass spectra obtained when the frequency of the AC high voltage was changed in the range of 20 kHz to 5 kHz measured at the threshold voltage for the field electron emission. Cl−, HCOO−, and CH3COO− product ions were formed by the reactions of O2−• with HCl, formic acid, and acetic acid contaminants, respectively, that are present in the laboratory air (see the latter section). There seems to be no noticeable frequency dependence on the field electron emission in the range of 5–20 kHz.
In addition to the use of a stainless steel acupuncture needle, various other metal needles were tested as electrodes. Metal wire, with a diameter of 0.1 mm, was cut tangentially by a nipper and then sharpened using Emery paper (# 1000) and was used for an emitter. Among the tested metals (Ti, W, Cr, Co, Mo, Pt, Pd, Fe, Au, Ni, Ir, Cu, constantan (Cu/Ni alloy), Pd/Pt(1/9)), Ti, Pd, constantan, Pd/Pt(1/9), and Cr were found to be appropriate for field electron emission. There is a crude trend that metals with lower work functions are better-suited as field electron emitters.
In our previous paper, an AC corona discharge was applied to an atmospheric-pressure chemical ionization (APCI) ion source for the first time.29) The AC corona discharge was found to be superior to a DC corona discharge for various reasons3,29): (i) corrosion of the needle electrode by the AC corona is much less than that for a DC corona, (ii) both positive and negative ions can be detected without changing the polarity of the high voltage power supply, (iii) an AC corona gives as strong positive and negative ion signal intensities as a DC corona even though an intermittent plasma is generated in the AC corona, (iv) ionization by an AC corona is milder than that for a DC corona, (v) transition to arc discharge for an AC corona is largely suppressed compared to that for a DC corona. These characteristic differences between AC and DC corona discharges can be envisaged by the observation of positive-mode mass spectra obtained by AC and DC corona discharges. Figure 6 shows the positive-mode mass spectra for ambient air measured under the same experimental conditions as in Fig. 4. As suggested in Fig. 4(b), the corona discharge started at the threshold voltage of ±1250 V in the positive-mode for the AC corona discharge in Fig. 6(a). The signal intensities for [(H2O)n+H]+ (n=2, 3) increase only gradually with increasing AC voltage from ±1250 V to ±1600 V. In contrast, for the DC corona discharge as shown in Figs. 6(d)–6(f), the ion signal intensities increase steeply with increasing applied DC voltage from the threshold voltage of +1900 V to +2100 V. With a further increase in +DC high voltage, a transition to arc discharge was anticipated. It should be noted that the threshold voltage for the DC corona discharge (+1900 V) was much higher than that for the AC corona discharge (±1250 V). This indicates that AC and DC corona discharges are based on quite different breakdown mechanisms. Plasma is an electrically conducting media composed of positive and negative charges and is generated by an electron avalanche induced by electrons accelerated in a high electric field in an insulating media. In the positive-mode DC discharge, nascent electrons that trigger the discharge are generated accidentally by cosmic rays or photoelectrons. The greater fraction of the nascent electrons are attracted to the anode, where they are annihilated by the high electric field near the anode. Due to the paucity of electrons that act as the seeds for gas discharge breakdown, a high threshold voltage is necessary for initiating a positive-mode DC corona discharge. In addition, the incidental generation of nascent electrons may lead to the positive-mode corona discharge being unstable. Owing to the application of a positive potential to the anode for the positive-mode DC corona, positive ions accumulate near the tip of the needle electrode leading to the formation of a Debye sheath that shields the potential applied to the needle. This explains why a high positive potential is necessary for maintaining a stable positive-mode DC corona discharge. In contrast, in the AC corona discharge, electrons are supplied to the insulating medium by the field electron emission in the negative-phase voltage, enabling the maintenance of discharge with a much lower applied voltage. In summary, the AC corona discharge can be maintained with a much lower voltage than DC corona discharge for both positive- and negative-mode of mass spectrometric operation.

A negative-mode corona discharge is beneficial for the detection of molecules that have positive electron affinities because all electrons are eventually converted into negative ions by electron attachment reactions such as the formation of O2−• in this experiment.
As described in the introduction, CO3−• causes oxidative damage to biological systems such as DNA and proteins. As shown in Fig. 4, a corona discharge generates reactive oxidants of O2−•, CO3−•, O3, etc. There are many commercially available household appliances that use a corona discharge for the sterilization of bacteria and virus in air. To examine the kinds of ions that are formed by sterilizers that use a corona discharge, the plasma-activated air flowing out from a commercial air sterilizer (USB type, Air Success Mini, Air Success, Kanagawa, Japan) was measured. Figure 7 shows a mass spectrum for air ionized by the negative DC mode multiple-ring corona discharge that is installed in the Air Success Mini. The mass spectrum is very similar to those shown in Figs. 4(e)–4(h) and oxidative CO3−• was detected as one of the major ions.

The reactions of O2−• with hydrocarbons, alcohols, acetone, and acetonitrile were examined by placing 10 μL liquid samples in the heater shown in Fig. 1(b). Neither H• nor H+ abstraction reactions were observed for these compounds. However, when 10 μL formic acid, acetic acid, and trifluoroacetic acid were introduced into the ion source, the respective deprotonated carboxylate ions HCOO−, CH3COO−, and CF3COO− were clearly detected as the major ions. After these three measurements, a mass spectrum for laboratory air contaminated by these three acids was collected, as shown in Fig. S7. All three acids were clearly detected indicating that the present field-electron-emission type ion source is suitable for the detection of trace amounts of acids. Deprotonated ions were also detected for several amino acids (leucine, isoleucine, alanine, and phenylalanine). Figures 8(a) and 8(b) show the mass spectra before and after introducing phenylalanine (Phe) into the ion source. Approximately 10 s after the deposition of a 10 μL aqueous solution of a 10−3 M phenylalanine on the heater at 140°C, deprotonated [Phe−H]− and [Phe+O2]−• cluster ions started to be detected. The ion signals continued to be detected for much longer than 10 s due to the slow evaporation of the phenylalanine at 140°C (melting point: 283°C, boiling point: 295°C). Fig. S8 shows the results obtained for nitric acid. Fig. S8(a) shows the mass spectrum before sample introduction, in which O2−• is detected as the only major ion. Fig. S8(b) shows the mass spectrum obtained when a cotton ball that was wetted by a 30% aqueous nitric acid was positioned in close proximity to the ion source at 0.24 min. O2−• was completely converted into NO3− and [HNO3+NO3]−. Figs. S8(c)–S8(e) show EIC for O2−•, NO3−, and [HNO3+NO3]−, respectively. NO3− was detected for hours after the sample introduction.

Table S2 summarizes the enthalpy changes for proton transfer reactions of O2−•, HCO3−, and CO3−• with acetic, formic and nitric acids.25,26) While all reactions are exothermic for O2−•, those for HCO3− and CO3−• are endothermic except for nitric acid. This is due to the fact that the proton affinity of O2−• (353 kcal mol−1) is much larger than that for CO3−• (333 kcal mol−1) and of HCO3− (338.7 kcal mol−1)25,26) (see Scheme 1).
In gas-phase reactions of CO3−•, O−• transfer, O2−• transfer, and H+ abstraction reactions with inorganic and organic molecules have been studied to date. However, H• abstraction reactions with organic molecules, although of interest, have not been reported. In this work, occurrence/nonoccurrence experiments of H• abstractions of CO3−• with various molecules in the gas phase are reported for the first time. H• abstraction was observed for n-hexane, cyclohexane, methanol, ethanol, 1-propanol, 2-propanol, and toluene, but no reactions were observed for acetonitrile, acetone, benzene, and H2O. DFT calculations clearly demonstrated the reason for this contrast between the occurrence for toluene and n-hexane and the nonoccurrence for benzene. In biological systems, CO3−• is capable of causing serious oxidative damage to proteins and DNA molecules via H• abstraction reactions. It should therefore be assumed that air sterilizers with the function via the use of a corona discharge ion source evolve CO3−• ions as the major ions, which could be harmful to mucous membranes such as lungs.
When an AC high voltage was applied to the sharp metal needle electrode in ambient air, tunneling electron emission from the tip of the needle was observed and the generation of electrons were detected as O2−• by an electron attachment reaction. O2−• did not show any reactivity toward hydrocarbons or alcohols but it abstracts H+ from acid molecules such as formic acid, acetic acid, nitric acid and amino acids. By investigating the threshold behavior of ion formation for AC and DC corona discharges, the reason why an AC corona is milder than a DC corona has been elucidated.
Mass Spectrom (Tokyo) 2021; 10(1): A0100