2022 Volume 11 Issue 1 Pages A0103
2022 Volume 11 Issue 1 Pages A0103
Electrospray ionization (ESI) mass spectrometry of transferrin can be used to diagnose congenital disorders of glycosylation (CDG) by detecting abnormal N-glycosylation due to reduced site occupancy or processing failure. Time-of-flight mass spectrometers are widely used to separate 25–45 charged ions in the m/z 1,700–3,000 range, and a summed zero-charge mass distribution is generated despite the risk of improper deconvolution. In this study, the low m/z region of the multiply-charged ion mass spectrum enabled a robust analysis of CDG. A triple quadrupole mass spectrometer, the standard instrument for newborn screening for inborn errors of metabolism, permitted the identification of the key ions characteristic of different types of CDG affecting PMM2, ALG14, SLC35A1, SLC35A2, MAN1B1 and PGM1 in the m/z 1,970–2,000 region. Charge deconvolution was used as a complementary tool for validating the findings. It was necessary to set a cutoff level for the evaluation, since small peaks indicating glycosylation failure or reduced sialylation were observed, even in control subjects. This method and workflow facilitates the implementation of MS-based analyses and the screening of CDG in clinical laboratories.
Congenital disorders of glycosylation (CDG), an expanding group of genetic diseases related to the glycosylation of proteins and lipids, are caused by mutations in over 140 genes.1) CDG are diagnosed by genetic and glycoprotein analysis of the patients with non-specific clinical conditions such as epilepsy and developmental delay.2) Transferrin, an 80 kDa glycoprotein, is the best analyte for detecting abnormal N-glycosylation due to its low level of microheterogeneity. It contains two N-glycosylation sites that are occupied by biantennary oligosaccharides with terminal N-acetylneuraminic acid (NeuAc, or sialic acid) units. Fully sialylated transferrin or tetrasialo-transferrin is the major (>90%) isoform in the serum of control subjects. Historically, abnormal glycosylation in CDG was classified into two types, i.e., CDG-I and II, based on isoelectric focusing (IEF) patterns. A lack of a glycan at one or two glycosylation sites produces disialo- or asialo-transferrin, which is referred to as CDG-I. Immature or partially truncated glycans that form CDG-II are characterized by the presence of trisialo- and monosialo-transferrin in IEF. This classification is still in use, because these types represent defective stages in the N-glycosylation pathway; CDG-I is derived from a defect in the formation of glycans on a membrane-anchored lipid dolichol or the subsequent en bloc transfer of the dolichol-linked glycans to nascent proteins in the endoplasmic reticulum (ER), and CDG-II occurs in the latter process of glycan maturation in the ER and the Golgi apparatus.
Mass spectrometry (MS) has a distinct advantage over IEF since its first application to CDG in 1992,3) when the electrospray ionization (ESI) MS of transferrin from CDG-I patients demonstrated that the “disialo-transferrin” was a molecule lacking one entire glycan but not a form that contained truncated glycans. Subsequently, it was found that MS could be used to characterize an alteration in the glycoprofile,4) namely the relative abundance of each glycoform, characteristic of CDG-II. With these excellent capabilities, MS has now become the key tool for diagnosing CDG.5,6)
ESI of transferrin generates multiply-charged ions bearing 25–45 protons, therefore requiring a wide mass range for mass separation up to m/z 3,000. For mass range and sensitivity reasons, a quadrupole time-of-flight (QTOF) type of mass separator is widely used for CDG.7) On the other hand, there are few reports of the use of quadrupole (Q) type mass analyzers for this purpose.8,9) Triple Q type mass spectrometers are the most popular MS instruments in clinical laboratories. While they are routinely employed in newborn screening for inborn errors of metabolism (IEMs), the mass scan range is often limited up to m/z 2,000. In this study, transferrin samples from CDG patients were analyzed by ESI-Q mass spectrometry. The limited mass range was not a critical weakness for the diagnosis of CDG, and a narrow mass range of less than m/z 2,000 showed sufficient capacity to permit different types of CDG to be identified.
Sera without personally identifiable information were obtained from the doctors in charge of the patients at the Osaka Women’s and Children’s Hospial (OWCH) for the diagnosis of CDG. The patients were affected by multisystem diseases of unknown etiology. A genetic diagnosis was made before or after the molecular abnormality was identified by MS. This study has been approved by the institutional review board of OWCH.
Immunopurification of transferrinImmunopurification was performed according to a previously reported method.10) Briefly, an affinity column was prepared using a rabbit (DAKO, Glostrup, Denmark) or a goat (Invitrogen, Thermo-Fisher Scientific, Waltham, MA, USA) polyclonal antibody against human transferrin and a ligand-coupling Sepharose column (HiTrap NHS-activated HP, GE Healthcare, Piscataway, NJ, USA), and the antibody-coupled Sepharose was then recovered from the column. A 10-μL portion of serum was mixed with a 20-μL slurry of the antibody-coupled Sepharose in 0.5 mL of phosphate-buffered saline (PBS), and the solution was incubated at 4°C for 30 min. After washing with PBS, the transferrin was eluted from the Sepharose with 0.1 M glycine–HCl buffer at pH 2.5.
Mass spectrometryLiquid chromatography MS was carried out by an API4500 ESI-triple Q mass spectrometer (Sciex, Framingham, MA, USA) or a QSTAR ESI-QTOF mass spectrometer (Sciex) connected to a C4 reversed phase column (2 mm diameter and 10 mm length, GL Sciences, Tokyo, Japan). After sample injection, the column was washed with 0.1% formic acid at a flow rate of 0.2 mL/min, and then eluted with 60% acetonitrile/0.1% formic acid at a flow rate of 0.05 mL/min.
API4500 was operated in the positive Q1 MS mode with the optimized parameters as follows: gas temperature was at 150°C, curtain gas pressure was 10 psi, ion source gas pressure was 16 psi, IonSpray voltage was 5.5 kV, declustering potential was 150 V, and entrance potential was 10 V. The full scan range was set from 1,780 to 2,000, and the scan rate was 10 Da/s. Polypropylene glycol was used for mass calibration.
QSTAR was operated in the positive TOF MS mode. The optimized parameters were as follows: gas temperature was ambient, nebulizer gas (GS1) was 40 psi, curtain gas pressure was 50 psi, IonSpray voltage was 5.5 kV, and declustering potential was 100 V. The full scan range was set from 1,500 to 3,000. The pulser frequency was 5 kHz and pulse duration was 20 μs. Sodium trifluoroacetate was used for mass calibration.11)
The zero-charge mass spectrum was generated by the Promass protein deconvolution software described above.
Statistical analysisStatistical analysis was performed by using JMP statistical analysis software (SAS Institute, NC, USA).
Human serotransferrin (UniProt accession number P02787) is composed of 679 amino acid residues. The molecular mass (average mass) of the unglycosylated transferrin is 75,156.9 (C3306 H5096 N912 O1002 S47), when 19 disulfide bonds are present. In the UniProt record, the sequence of a predominant electrophoretic variant, C1 or TF*C1, contains isoleucine at residue 448. However, according to the public-domain archive for human single nucleotide variations, dbSNP (rs2692696), the residue is valine in the Japanese and other ethnic groups in the world, and therefore the molecular mass is 75,142.9 (C3305 H5094 N912 O1002 S47). The major (>90%) glycoform of serum transferrin is disialylated biantennary oligosaccharide (C84 H136 N6 O61; 2,206.0 for average mass), and it is partially fucosylated.12) In addition, a small fraction of transferrin has a triantennary oligosaccharide. The molecular mass of serum transferrin with two disialylated biantennary glycans, or tetrasialotransferrin, is 79,554.9 (C3473 H5366 N924 O1124 S47). The theoretical isotope pattern, as calculated by enviPat Web 2.4 (https://www.envipat.eawag.ch/)13) at different resolutions is shown in Fig. 1A. Distribution of the isotopic cluster is wide, and the peak width at a half maximum is approximately 15, 20, 25, and 45 Da at resolutions of 100,000, 10,000, 5,000, and 2,000, respectively. The ESI mass spectrum of a sample of transferrin obtained from a control subject was acquired by a QTOF mass spectrometer with a resolving power of approximately 5,000 (Fig. 2A), and the [M+40H]40+ ion was charge-deconvoluted (Fig. 1B). The peak width was 38 Da, which was larger than the theoretical value of 25 Da. The ESI-Q mass spectrum was then acquired by an instrument whose mass range was limited to m/z 2,000 for specific use in IEMs screening (Fig. 2B). The resolution was approximately 2,000 at m/z 1,800–2,000. The peak width of the charge-deconvoluted [M+40H]40+ ion of the ESI-Q mass spectrum was 52 Da (Fig. 1C). The peak broadening, observed in both QTOF-MS and Q-MS, would be expected to depress the accuracy owing to ambiguous reading of the peak centroid or the overlapping of unknown peaks adjacent to the target.


https://doi.org/10.50893/data.massspectrometry.19446803
The major molecular or glycoform phenotypes of transferrin observed in CDG are illustrated with their masses in Fig. 3. It should be noted here that the mature/normal glycoform, disialylated biantennary oligosaccharide, is present in CDG and is the predominant form with the exception of some CDG-I type diseases. Since glycosylation is a post-translational modification, the core protein sequence is the same for normal and abnormal species in each patient. This helps in characterizing aberrant glycoforms based on the size of the mass shift from normal species. As shown in Fig. 3, the mass difference between the mature glycoform(s) and other forms exceeds 100 Da, suggesting that ESI-MS, either by QTOF or Q, can detect a transferrin molecule bearing abnormal glycoforms. Indeed, they were separated from each other in the ESI-QTOF mass spectra shown in Fig. 4.


The deconvolution program is a tool that allows the ESI mass spectrum of multiply-charged ions to be transformed into a summed zero-charge mass distribution. Deconvolution aids in interpretation but a proper parameter setting is required to generate a valid spectrum, thus risking pitfalls.10) For example, a small but diagnostic peak of CDG-I disappeared after a small change in the parameters (Supplementary Figure S2). Furthermore, in the ESI mass spectrum of PMM2-CDG, the m/z values of [M+36H]36+ ions of normal transferrin, [M+35H]35+ ions lacking one glycan, and [M+34H]34+ ions lacking two glycans were found to be 2,210.9, 2,211.0, and 2,211.1, respectively, and are completely overlapped (Figs. 5A and 1S). Similarly, the peaks for [M+33H]33+ ions lacking one glycan at m/z 2,344.9 and for [M+34H]34+ ions of fucosylated normal transferrin at m/z 2,345.1 were very close (Fig. 1S). Dealing with these overlapping ions would be challenging. On the other hand, the original multiply-charged ion mass spectrum in the lower mass region was free from overlapping, and the key glycoforms characterizing various types of CDG were detectable in a range below m/z 2,100 (Figs. 5B and 6).

https://doi.org/10.50893/data.massspectrometry.19446827

https://doi.org/10.50893/data.massspectrometry.19446833
These findings suggest that a reliable analysis is possible even with a mass spectrometer with a limited mass range. Various types of CDG were analyzed by a Q mass analyzer whose mass range was limited up to m/z 2,000 (Fig. 7). Key molecules characterizing CDG were identified, and the forms characterized as CDG-I and CDG-II type diseases were discriminated against each other in a mass window from m/z 1,970 to m/z 2,000. As shown in Fig. 8, the peaks at m/z 1,984.3 and m/z 1,982.6 were 39-charged “b” and 40-charged “x,” respectively. Form “b” lacks one glycan, indicating reduced site occupancy characteristics of CDG-I. Form “x” lacks one sialic acid and is the common molecular phenotype in many CDG-II diseases.

https://doi.org/10.50893/data.massspectrometry.19446836

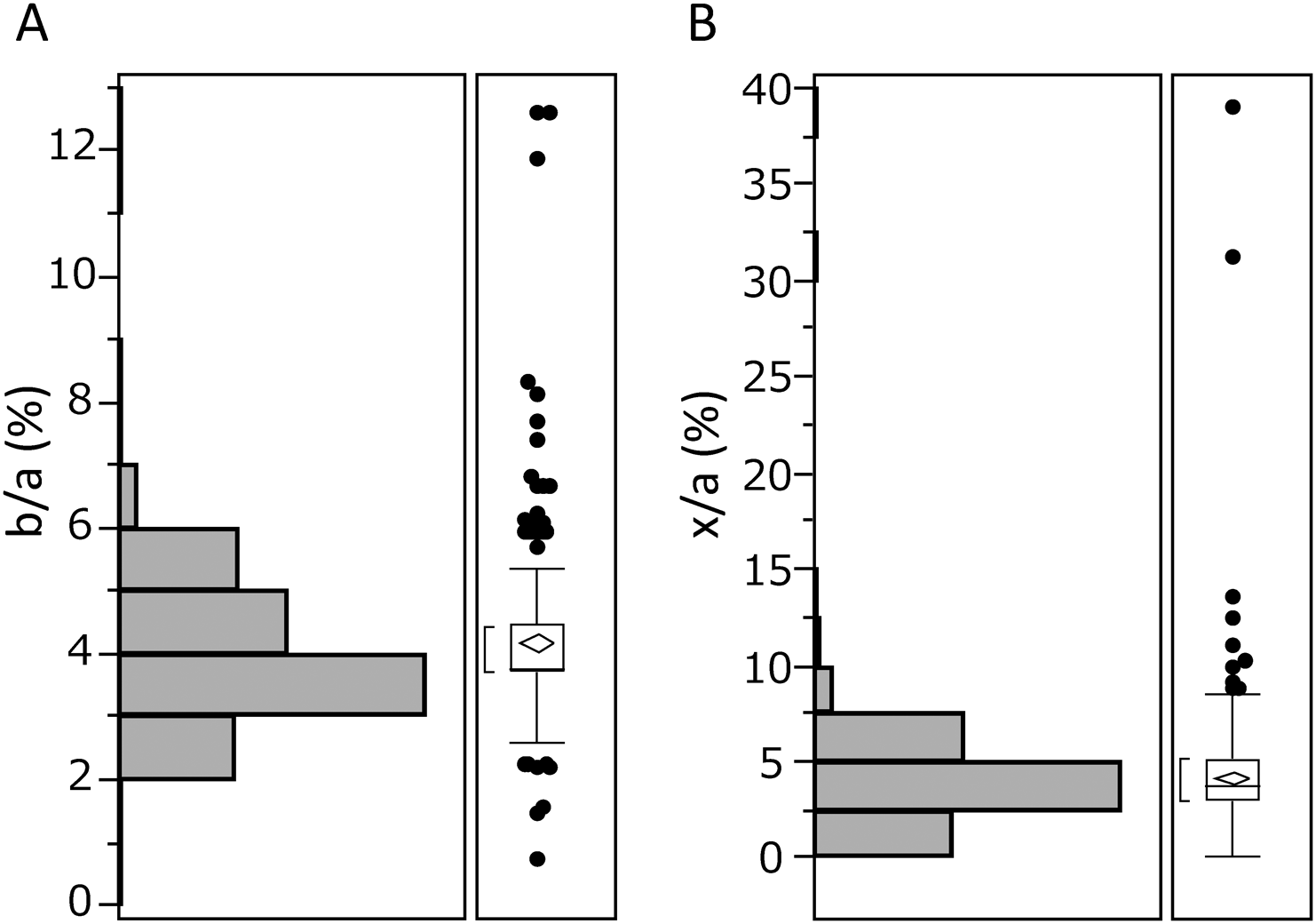
As shown in Fig. 8, sample from the control subjects also showed small peaks corresponding to “b” and “x,” while peaks “y” and “z” were not observed, indicating that a statistical evaluation would be needed to define or diagnose “abnormal.” To this end, the ratio of the intensity of these peaks to peak “a” at m/z 1,989.9 was calculated in a cohort of undiagnosed 331 samples (Fig. 9). The interquartile range of the b/a ratio was 3.7–4.5% and the upper limit of the reference range was 5.3% when calculated using 90% sample quantiles, and that of the x/a ratio was 3.0–5.2% and the upper limit was 6.7%. The increased ratios of b/a and x/a suggest CDG-I and II, respectively, and patients whose value exceeds the upper limit would be candidates for genetic analysis. For reference, the data for the genetically diagnosed CDG patients are presented in Table 1. The form “b” in PMM2- and ALG14-CDG and the form “x” in SLC35A1- and SLC35A2-CDG were significantly increased. Increases in both “b” and “x” in PGM1-CDG indicate a mixed CDG-I/II type. The deconvoluted spectra were helpful for verifying the presence of these key forms (Supplementary Figure S3).
| CDG type | b/a | x/a |
|---|---|---|
| PMM2 | 123.0* | 0 |
| ALG14 | 8.8* | 1.5 |
| SLC35A1 | 2.3 | 21.1* |
| SLC35A2 | 1.5 | 16.2* |
| PGM1 | 27.3* | 7.8* |
| MAN1B1 | 0 | 0 |
The calculation is based on the ESI-Q mass spectrum in Fig. 7.
*significantly increased.
The molecular mass of transferrin cannot be determined accurately due to the wide distribution of isotope clusters. Molecular phenotyping of CDG can also be achieved in the low m/z part of the multiply-charged ion mass spectrum. The aberrant molecules that are characteristic of various types of CDG were identified in the m/z 1,970–2,000 range of the ESI-Q mass spectrum. Even in control subjects, there were small peaks indicative of glycosylation failure or reduced sialylation, so it is necessary to set a cutoff level in order to achieve a valid evaluation.
Special thanks are extended to M. Kadoya for technical assistance. This work was supported by a Grant-in-Aid from AMED (JP19ek0109418).
Mass Spectrom (Tokyo) 2022; 11(1): A0103