2013 年 2 巻 1 号 p. A0019
2013 年 2 巻 1 号 p. A0019
The matrix-assisted laser desorption/ionization in-source decay (MALDI-ISD) of peptides and glycans was studied using an oxidizing chemical, 5-nitrosalicylic acid (5-NSA) as the matrix. The use of 5-NSA for the MALDI-ISD of peptides and glycans promoted fragmentation pathways involving “hydrogen-deficient” radical precursors. Hydrogen abstraction from peptides resulted in the production of a “hydrogen-deficient” peptide radical that contained a radical site on the amide nitrogen in the peptide backbone with subsequent radical-induced cleavage at the Cα–C bonds. Cleavage at the Cα–C bond leads to the production of an a•/x fragment pair and the radical a• ions then undergo further hydrogen abstraction to form a ions after Cα–C bond cleavage. Since the Pro residue does not contain a nitrogen-centered radical site, Cα–C bond cleavage does not occur at this site. Alternatively, the specific cleavage of CO−N bonds leads to a b•/y fragment pair at Xxx−Pro which occurs via hydrogen abstraction from the Cα−H in the Pro residue. In contrast, “hydrogen-deficient” glycan radicals were generated by hydrogen abstraction from hydroxyl groups in glycans. Both glycosidic and cross-ring cleavages occurred as the result of the degradation of “hydrogen-deficient” glycan radicals. Cross-ring cleavage ions are potentially useful in linkage analysis, one of the most critical steps in the characterization of glycans. Moreover, isobaric glycans could be distinguished by structure specific ISD ions, and the molar ratio of glycan isomers in a mixture can be estimated from their fragment ions abundance ratios. MALDI-ISD with 5-NSA could be a useful method for the sequencing of peptides including the location of post-translational modifications, identification and semi-quantitative analysis of mixtures of glycan isomers.
Because of its high sensitivity and ease of use, mass spectrometry (MS) represents a powerful analytical tool that can be applied in a wide variety of scientific fields. In particular, matrix-assisted laser desorption/ionization (MALDI)1) is recognized as an indispensable analytical method for identifying peptides and proteins. One of the main advantages of MALDI is that single charged intact ions without abundant fragmentation can be produced. To date, peptide-mass fingerprinting (PMF) with MALDI-MS is a common approach for the characterization of isolated proteins by the analysis of peptide digests.2,3) MALDI analysis following MS/MS by collision-induced dissociation (CID) provides useful information for the characterization of proteins. CID is a commonly available fragmentation method that is applicable to closed shell ions.4) Briefly, the collision energy deposited on a peptide ion is converted into vibrational energy that is distributed over the entire ion, leading to the cleavage of the lowest energy bond (usually labile or peptide bonds). Therefore, proteins exhibit a low fragmentation efficiency by CID due to the less the energy received per degree of freedom. CID is not applicable for use in a top-down sequencing which is used for the direct fragmentation of an intact protein in the mass spectrometer without any enzyme digestion.
For a “top-down” approach, alternative fragmentation methods such as MALDI in-source decay,5) electron capture dissociation (ECD)6) and electron transfer dissociation (ETD)7) leading to the fragmentation of radical ions, can be used to characterize proteins. The advantages in the “top-down” approach are a high throughput, straightforward methodology and the need for a small amount of sample.8,9) Additionally, they could be useful methods for de novo protein sequencing, including identifying post-translational modifications.8,9)
MALDI in-source decay (ISD) is a type of fragmentation that occurs rapidly in the MALDI source, after the laser shot and before ion extraction. Hydrogen transfer from the matrix to a carbonyl oxygen on peptide backbone initiates MALDI-ISD via the formation of “hydrogen-abundant” peptide radicals.10) A strong signal of ISD ions is observed when laser shots are performed on matrix crystals, whereas the formation of ISD ions is suppressed when an ionic liquid matrix is used.11) This indicates that the hydrogen transfer between peptide and matrix which is an initial step of MALDI-ISD mainly occurs on the matrix crystal during the dissipation of the laser energy and before desorption.11) Subsequently, the resulting “hydrogen-abundant peptide” leads to the formation of c'/z• fragments pair by radical-induced cleavage and then z• ions gain a hydrogen radical or react with a matrix radical12) (Scheme 1a). The z• fragments can undergo radical reactions and subsequent degradation, because the reactivity of radical z• fragments is higher than that of c' fragments.13,14) Additionally, an a•/y' fragment pair is also produced via the formation of “hydrogen-abundant” peptide radicals (Scheme 1b). Hydrogen attachment of the side-chain in cysteine residues also leads to radical-induced cleavage at the N–Cα bond located on the left side (N-terminal direction) of the cysteine residue.15)

Previously, 2,5-dihydroxybenzoic acid (2,5-DHB) was widely used in MALDI-ISD experiment.16) Recently, a number of matrices have been developed to increase the performance of MALDI-ISD for peptides and proteins. In particular, 1,5-diaminonaphtalene (1,5-DAN)17) and 2-aminobenzoic acid (2-AA)18) were reported to efficiently induce the MALDI-ISD of peptides and proteins. MALDI is well suited for use with axial time-of-flight (TOF) mass spectrometers.16) In contrast, MALDI-ISD combined with Fourier transform-ion cyclotron resonance mass spectrometer (FTICR MS) which provides high-mass resolution and sub-ppm mass accuracy measurements has been developed.19) MALDI-ISD with a FTICR MS is useful method for biomarker validation as a complement to classical histology.
ECD and ETD are positive polarity electrospray ionization-based radical-induced fragmentation methods, initiated by the association of electrons with multiply protonated analytes. Backbone cleavage by MALDI-ISD and ECD/ETD is mechanistically similar, and involves the formation of “hydrogen-abundant” peptide radicals and subsequent radical-induced N–Cα bond cleavage.20) Zubarev et al. also developed an electrospray-based fragmentation technique operating in the negative polarity, electron detachment dissociation (EDD), which causes the ejection of an electron from the multiple deprotonated peptide by using a fast electron (>10 eV).21)
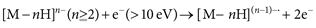 |
In contrast to EDD, negative electron transfer dissociation (NECD)22) involves the abstraction of an electron from the multiple deprotonated peptide by using a radical cation, such as Xe22) and fluoranthene (C16H10).23)
 |
EDD/NECD is negative ion analogue of ECD/ETD, however, the mechanism for various types of backbone cleavages are different from one another. Electron detachment results in the formation of a charge-reduced peptide anion that contains a radical site on the carboxyl group of the side-chain or C-terminal carboxyl group. Subsequently, a nitrogen-centered radical product is formed via hydrogen transfer from the backbone amide nitrogen to the radical site on the carboxyl group. The radical on the amide nitrogen induces dissociation of the Cα–C bond, leading to the formation of an a•/x fragment pair.24,25)
 |
Additionally, EDD and NECD can provide useful information for identification of acidic glycans.26–28)
Femtosecond laser-induced ionization/dissociation (fs-LID)29,30) has been developed for identifying singly-charged peptide. Fs-LID causes the detachment of an electron from singly-protonated analytes [M+H]+ by femtosecond laser irradiation, forming a charge-induced peptide cation [M+H]2+•. Subsequently, the radical induces the dissociation of the Cα–C bond, leading to an a•/x fragment pair.
 |
 |
As described above, backbone cleavage by EDD, NECD, and fs-LID is mechanistically similar, leading to the formation of “hydrogen-deficient” peptide radicals and subsequent radical-induced Cα–C bond cleavage. EDD/NECD and fs-LID is applicable to only multiple-deprotonated peptide and singly-protonated peptide, respectively.
More recently, we found that the use of an oxidizing matrix, 5-nitrosalicylic acid (5-NSA) generated “hydrogen-deficient” peptide and glycan radicals during the MALDI-ISD process.31–36) In this article, I focus on the mechanisms involved in the fragmentation of peptides and glycans in MALDI-ISD with 5-NSA. The usefulness of MALDI-ISD for the sequencing of phosphopeptides and identification/semi-quantification of glycan isomers is also discussed.
NotationHerein the unambiguous notation of Zubarev when naming the peptide fragment ions is used.20) According to this notation, homolytic Cα–C bond cleavage yields the radical fragments a• and x•, and loss of a hydrogen atom from an a• or x• fragment produces an a or x fragment, respectively. The product of the transfer of a hydrogen atom to an a• or x• fragment is denoted as a' and x', respectively. Unless noted otherwise, all assigned peaks represent singly protonated molecules [M+H]+ and deprotonated molecules [M−H]− in the positive ion mode and the negative ion mode, respectively.
For glycan fragmentation, the notation of Domon and Costello is employed, as shown in Scheme 2.37) Unless otherwise noted, all assigned peaks represent singly sodiated molecules, i.e., [M+Na]+.

The choice of a matrix for MALDI-ISD can dramatically affect the observed ISD fragment ions and the quality of the mass spectrum. 2,5-DHB is widely used in MALDI-ISD experiments and seems to be one of the best matrix candidates available for the formation of “hydrogen-abundant” peptide radicals in MALDI-ISD.38) The 5-hydroxyl group of 2,5-DHB plays an important role in the formation of “hydrogen-abundant” peptide radicals.10,38) My focus was on the nature of the functional group at the 5-position in salicylic acid derivatives, and an oxidizing chemical, 5-NSA was used for the MALDI matrix.31) Figure 1 shows a comparison of positive-ion MALDI mass spectra of ACTH18–35 (RPVKVYPNGAEDESAEAF) obtained with two different matrices 2,5-DHB and 5-NSA. When 2,5-DHB was used, c' ions accompanied by a ions were generated (Fig. 1a). As described above, an a•/y' fragment pair might be formed by fragmentation of a “hydrogen-abundant” peptide radical as well as the formation of a c'/z• fragment pair11) (Scheme 1b). In contrast, the use of 5-NSA generated a ions with strong signal intensities and no c' ions were generated (Fig. 1b). Therefore, the formation of a ions with 5-NSA occurs via a different in mechanism from that when 2,5-DHB is used. Figure 2 shows enlarged spectra for [M+H]+ of ACTH18–35 with 2,5-DHB and 5-NSA. It should be noted that the use of 5-NSA gave dehydrogenated or oxidized ACTH18–35, [M–2H+H]+, which is absent in the MALDI-ISD spectra when 2,5-DHB is used. The oxidized product [M–2H+H]+ was formed by hydrogen transfer from peptide molecules to the nitro group in 5-NSA. The abstraction of hydrogen atoms from N–H and Cα–H bonds on the peptide backbone results in the formation of oxidized peptides, [M–2H+H]+ (Scheme 3). Therefore, it appears that a ions in MALDI-ISD using 5-NSA are generated accompanied with hydrogen abstraction.



To ascertain the most probable pathway for the formation of a ions in MALDI-ISD with 5-NSA, a deuterium-labeling peptide (RLGNQWA(d3)VG(d2)DLAE) was conducted as a model and a synthetic peptide (RLGNQWAVGDLAE) as a control in a MALDI-ISD experiment with 5-NSA as the matrix.31) The difference between those peptides is that the β-hydrogens at Ala7 and the α-hydrogens at Gly9 are substituted with deuterium. As shown in the a9 ion region of the mass spectra, a mass shift of 5 Da was observed in these a9 products (Fig. 3). This mass shift is consistent with the number of deuterium labels in the Ala7 (CβD3) and Gly9 (CαD2). This indicates that the a ions observed in the MALDI-ISD spectra with 5-NSA are formed via the abstraction of the amide hydrogen on the peptide backbone, as shown in Scheme 4.


In order to address the most probable pathway for the formation of a ions via hydrogen abstraction, MALDI-ISD experiments were conducted using [Arg18]-ACTH19–36 (PVKVYPNGAEDESAEAFR). The only difference between ACTH and [Arg18]-ACTH19–36 is the position of the Arg residue, which has the highest proton affinity among the most common 20 amino acid residues. The presence of a positive charge on the ISD fragments is necessary for their observation in a positive-ion MALDI-ISD spectrum. It has previously been reported that peptides containing an Arg residue near the N-terminus preferentially produce N-terminal side fragment ions, while the presence of an Arg residue at the C-terminal favors the formation of C-terminal side fragment ions.39) As expected, the MALDI-ISD spectrum of [Arg18]-ACTH19–36 shows x ions, which are counterpart of a ions (Fig. 4). 5-NSA give principally a ions of ACTH18–35 and x ions of [Arg18]-ACTH19–36 which is indicative of cleavage at the Cα–C bonds. Consequently, MALDI-ISD with 5-NSA is initiated by the transfer of a hydrogen radical from an amide group on the peptide backbone to the 5-NSA molecule, leading to the formation of a “hydrogen-deficient” peptide radical. The Cα–C bond of the peptide backbone is subsequently cleaved. Upon forming this “hydrogen-deficient” peptide, two Cα–C bond cleavage pathways producing either a•/x or a/x• fragments pairs are theoretically possible. However, all ISD fragment ions contain an even-number of electrons, i.e., a and x ions. Therefore, it is important to determine the mechanism of cleavage that results in the formation of a and x ions. It should be noted that the x13 ion derived from the cleavage of the Cα–C bond at Xxx–Pro was absent, probably due to the absence of a nitrogen-centered radical site in the Pro residue. Cα–C bond cleavage at Pro–Xxx and Xxx–Pro bonds would lead to the formation of a•/x and a/x• fragment pairs, respectively (Scheme 5). The absence of a x13 ion from the cleavage of Cα–C bonds at Tyr–Pro in Fig. 4 indicates that fragmentation leading to a/x• fragments pair does not occur (Scheme 5b), which further suggests that the general mechanism proposed in Scheme 5 holds for Cα–C bonds that do not involve Xxx–Pro bonds. An ab initio calculations showed that the energy barrier for the formation of an a•/x fragment pair is lower and that the formation of an a•/x fragment pair is more favorable than that for an a/x• fragment pair.24,25) Therefore, the proposed Cα–C bond cleavage mechanism (Scheme 6) is supported by the ab initio calculations. However, radical fragment a• ions were not observed in MALDI-ISD spectra when 5-NSA was used, and a ions were detected instead. It is likely that the amounts of exited 5-NSA molecules and 5-NSA radicals in the MALDI plume are sufficient to form a ions via further hydrogen abstraction after the Cα–C bond cleavage (Scheme 6).



The abundance of a ion that originated from cleavage at the C-terminal side of Gly residue (Gly–Xxx) is small, e.g., a9 of ACTH18–35 (Fig. 1b), a9 of substance P, and a9 of synthetic peptide (RLGNQWAVGDLAE). Therefore, Cα–C bond cleavage at Gly–Xxx is less susceptible to fragmentation in MALDI-ISD with 5-NSA than are those of other residues. If thermochemistry were the driving force for the Cα–C bond cleavage, the cleavage efficiency would be expected to depend on the bond strength which is correlated with the stability of a•/x fragment pairs. The stability of radical a• fragment is lower than that for a closed-shell x fragment. Thus the bond strength decreases with increasing stability of the a• fragment, which can be considered as the stability of individual amino acid radical that contained a radical site on the α-carbon atom. According to the results of the ab initio calculations, the glycine radical shows lowest stability of the calculated 12 amino acids.40) When an a• fragment contained a Val side-chain at the radical site, the formation of an a•/x fragment pair from a hydrogen-deficient peptide radical is nearly thermoneutral.24) The enthalpy of formation of a glycine radical is 20–25 kJ/mol higher than that of a valine radical.40) As a consequence, the formation of an a•/x fragment pair from Gly–Xxx bond cleavage would be expected to be endothermic. The low abundance of a ion that originated from Gly–Xxx cleavage can be understood from low stability of the radical a• fragment.
As described above, the x13 ion arising from the cleavage at Tyr–Pro in the MALDI-ISD spectrum of [Arg18]-ACTH19–36 was absent but, instead, a y13 ion was observed (Fig. 4). Additionally, b6 ions originated from the cleavage at Tyr–Pro bonds were observed in the MALDI-ISD spectrum of ACTH18–35 (Fig. 1b). The cleavage of the CO–N bond at Xxx–Pro to form b and y ions occur with hydrogen abstraction from the Cα–H bond at a Pro residue (Scheme 7). In contrast, the a6 ion of ACTH18–35, which is formed by Cα–C bond cleavage at Tyr–Pro, was observed (Fig. 1). This a6 ion may be derived from the peptide bond cleavage at Tyr–Pro (Scheme 7). No evidence was found for the production of x ions produced as the result of cleavage at Xxx–Pro in the MALDI-ISD spectrum of other Pro-rich peptides such as bradykinin and bradykinin potentiator B, whereas the counterpart a ions were still observed.32,34) These results suggest that the a ions originating from the cleavage of Xxx–Pro are formed by the further degradation of b• ions (Scheme 7), indicating the competitive formation of b and a ions from b• ions during MALDI-ISD via hydrogen abstraction. MALDI-ISD also produced a snd b ions by the cleavage at N-terminal side of N-methylglycine, which contains methyl group instead of amide hydrogen.32) Proposed fragmentation process at N-terminal side of Pro (Scheme 7) is supported by the MALDI-ISD experiment of N-methylglycine containing peptide.

The utility of MALDI-ISD, in conjunction with 5-NSA for the sequencing of phosphopeptides is discussed in this section.33) I chose bovine β-casein tryptic peptides as model phosphopeptides, because they have frequently been used for evaluating the performance of MS instruments. In this experiment, β-casein was digested with trypsin and the resulting tetra-phospholylated tryptic peptide (RELEELNVPGEIVEpSLpSpSpSEESITR) was isolated from the digest by TiO2-based enrichment according to a previously published protocol.41)
The acidic phosphorylated peptides are selectively detected in negative-ion MALDI and MALDI-ISD then occurs by charge-remote fragmentation processes. Thus, this phosphopeptide was analyzed by both positive- and negative-ion MALDI-ISD. The positive- and negative-ion MALDI-ISD spectra of the tetra-phosphopeptide obtained using 5-NSA are shown in Fig. 5. The tetra-phosphopeptide having Arg residues at both the N- and C-termini would be expected to give rise to N- and C-terminal side fragment ions in positive-ion MALDI-ISD experiments. However, a ions were found to be dominant, as shown in Fig. 5a. It is likely that the presence of an N-terminal Arg residue facilitates the protonation reaction compared with the C-terminal Arg residue.42) In the negative-ion mode, the favored sites of deprotonation in the tetra-phosphorylated peptide are phosphate groups and Glu residues. x Ions were found to be dominant in negative-ion MALDI-ISD using 5-NSA (Fig. 5b), because the presence of phosphate groups near the C-terminus facilitates the deprotonation reaction at that site, rather than the Glu site. The x ion observed in the lowest m/z region is x7, which is the first fragment to contain a phosphorylated Ser residue. It is likely that the acidic phosphate group is effectively deprotonated, so that the negative charge is localized on phosphorylated Ser residues near the C-terminus. All of the a ions in Fig. 5a and x ions in Fig. 5b containing modified residues retained all four phosphate residues. The sequencing and precise determination of Ser15, Ser17, Ser18, and Ser19 as the sites of phosphorylation were accomplished by combining information from both positive- and negative-ion MALDI-ISD spectra using 5-NSA as the matrix. MALDI-ISD in conjunction with 5-NSA promises to be a useful method for the sequencing of peptides including determining the location of post-translational modifications.

In addition to phosphorylation, glycosylation is also an important regulatory covalent protein post-translational modification.43,44) MALDI-ISD can be used to determine the sites of glycosylation as well as phosphorylation.45) However, the focus here is on the structure analysis of glycans, because they directly affect the structure, stability and function of glycoproteins,46) and the structural study of oligosaccharides has become an important part of efforts to gain further insights into glycosylation. The structural analysis of glycans in glycoproteins generally involves their removal from glycoproteins, followed by analysis by MALDI-MS. Glycans can easily form sodium adducts because they have a high affinity for alkali cations. Positive-ion mode MALDI post-source decay (PSD) and MALDI MS/MS with low-energy CID are commonly used fragmentation methods for characterizing glycans.47) Low energy CID and MALDI-PSD of [M+Na]+ mainly lead to the cleavage of glycosyl bonds. In contrast, cross-ring cleavage fragments which are useful for a linkage analysis of glycans are rarely found in positive-ion low-energy CID and MALDI-PSD spectra.
As discussed above, hydrogen transfer between analyte and matrix is a feature of MALDI-ISD. Since most glycans do not contain unsaturated bonds, the formation of “hydrogen-abundant” glycan radicals is not efficiently occurred. Therefore, the use of 2,5-DHB does not lead to the radical fragmentation of glycans. In contrast, 5-NSA would be expected to produce “hydrogen-deficient” glycan radicals by hydrogen abstraction, leading to the formation of ISD fragments. In this section, the utility of MALDI-ISD with 5-NSA for the identification and semi-quantification of glycans is discussed.35) I chose Lacto-N-difucohexaose I (LNDFH-I) and Lacto-N-difucohexaose II (LNDFH-II) as model glycans (Scheme 8). The only difference between these glycans is the binding position of the fucose unit. These glycans are difficult to distinguish by conventional MS/MS analysis, such as MALDI-PSD of [M+Na]+.35)

Figure 6 shows a comparison of the positive-ion MALDI mass spectra of LNDFH-I and LNDFH-II obtained using 5-NSA as the matrix. The use of 5-NSA generated sodiated analytes [M+Na]+ and ISD fragment ions accompanied the oxidized product [M–2H+Na]+. This suggests that a “hydrogen-deficient” glycan radical [M−H]• is formed by hydrogen abstraction with subsequent radical induced cleavage, leading to ISD fragments. As shown in Fig. 6, the fragmentation patterns in the MALDI-ISD spectra of LNDFH-I and LNDFH-II are different, whereas the only difference between LNDFH-I and LNDFH-II is the binding position of fucose. Therefore, MALDI-ISD with 5-NSA represents a useful method for the identification of isobaric glycans.

In order to further investigate the ISD mechanism of glycans, we focused our attention on the transfer of hydrogen from glycans to 5-NSA molecules. Figure 7 shows the positive-ion MALDI mass spectrum of permethylated LNDFH-I with 5-NSA. The [M+Na]+ of permethylated LNDFH-I is observed as an intense signal, whereas the oxidized product [M–2H+Na]+ and ISD fragments were absent. This indicates that hydrogen abstraction from C–H does not occur and ISD fragments are formed via hydrogen abstraction from hydroxyl groups in glycans.

Therefore, a proposed pathway for glycosyl bond cleavage in MALDI-ISD with 5-NSA is shown in Scheme 9. Upon forming this hydrogen-deficient glycan radical, two glycosyl bond cleavage pathways, resulting in the production of either B/(Y–H)• or (C–H)•/Z fragment pairs are theoretically possible. However, the observed ISD fragments contain even-electrons, i.e., Y and C fragment ions as well as in the case of peptides. It is likely that Y and C fragments are formed via the attachment of hydrogen between hydroxyl or carboxyl groups in 5-NSA and (Y–H)• or (C–H)• fragments after glycosyl bond cleavage (Scheme 9).

In addition to fragments resulting from glycosylic bond cleavage, other fragments are also observed. Indeed, the ISD fragments for [M–C4H8O4+Na]+ at m/z 902.3 and [M–C2H4O2+Na]+ at m/z 962.3 are generated by cross-ring cleavage, as shown in the MALDI-ISD spectrum of LNDFH-I (Fig. 6a). The two isobaric fragments resulting from cross-ring cleavage can have the m/z 902.3, the 2,4A5 fragment at the reducing glucose residue or the 0,2X4α at the galactose residue. The proposed mechanism for the formation of 2,4A5 and 0,2X4α are shown in Scheme 10. To identify the structure of the ISD ion at m/z 902.3, pseudo-MS3 experiments performed, i.e., mass selection of ion at m/z 902.3 formed by MALDI-ISD following PSD, as shown in Fig. 8. The PSD of the ISD ions at m/z 902.3 gave a fragment ion at m/z 594.2, which originates by the loss of galactose and fucose. By contrast, the loss of glucose from this ISD ion was absent. Therefore, the ISD ion [M–C4H8O4+Na]+ were determined to be produced by cross-ring cleavage at the reducing terminal end 2,4A5. In a comparison of the MALDI-ISD spectra of LNDFH-I and LNDFH-II, it can be seen that this loss of C4H8O4 is not observed in the MALDI-ISD of LNDFH-II. The only difference between LNDFH-I and LNDFH-II lies in the position of the fucose unit. The loss of C4H8O4 from LNDFH-II is suppressed when the fucose is located at the reducing glucose residue.


For the ISD ion at m/z 962.3 (C2H4O2 loss from [M+Na]+), two cross-ring cleavage pathways for forming 1,3A5 and 0,4A5 are possible (Scheme 11a and 11b). The fragment ion at m/z 962.3 was absent in the mass spectrum of LNDFH-II, and instead a fragment at m/z 816.3 was observed. The signal at m/z 816.3 in the MALDI-ISD of LNDFH-II was identified as 1,3A4 which is formed by cross-ring cleavage including the fucose residue bound to the C-3 position of reducing glucose residue. Therefore, the cross-ring fragments at m/z 962.3 in MALDI-ISD of LNDFH-I could be assigned as 1,3A5. LNDFH-I also generated a fragment ion at m/z 816.3, which was identified as arising from cross-ring cleavage at the galactose residue, 1,3X4α. The formation of a glycan radical at the radical site on the C-1 position of galactose is impossible, because the galactose is bound to N-acetylglucosamine. Therefore, the formation of 1,3X4α is initiated by the abstraction of a hydrogen from the hydroxyl group at the C-4 position. Subsequently, radical induced cross-ring cleavage leads to 1,3X4α, as shown in Scheme 11c. In contrast, LNDFH-II would be expected to give 1,3X4 fragment at m/z 962.3. However, this fragment was absent in MALDI-ISD spectra of LNDFH-II. The formation of 1,3X4 fragment might be suppressed by the lack of fucose at galactose residue. However, fragmentation mechanism in MALDI-ISD with hydrogen abstraction is not yet fully understood. Nevertheless, the cross-ring cleavage fragments observed in MALDI-ISD with 5-NSA are very informative for identification of glycans.

When comparing MALDI-ISD spectra of LNDFH-I and LNDFH-II (Fig. 6), ISD ions at m/z 680.2 (B3), 842.3 (B4), 860.3 (C4), 902.3 (2,4A5), and 962.3 (1,3A5) originated from LNDFH-I only. In contrast, ISD ions at m/z 511.2 (Y2α) and 568.2 (C3α/Y3α″) appeared only in the MALDI-ISD of LNDFH-II. The strong intensity of ISD ions at m/z 876.3, formed by the loss of fucose are observed in both MALDI-ISD spectra. The fragment ions observed in the MALDI-ISD spectra are summarized in Table 1.
| 511.2 | 534.2 | 550.2 | 568.2 | 680.2 | 696.2 | 714.2 | 816.3 | 842.3 | 860.3 | 876.3 | 902.3 | 962.3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LNDFH-I | — | Z3α/C4 | Z3α/Y3β | — | B3 | Z3α | Y3α | 1,3X4α | B4 | C4 | Y3β, Y4α | 2,4A5 | 1,3A5 |
| LNDFH-II | Y2α | B2α | B3α/Y3α″ | C3α/Y3α″ | — | B3α | C3α | 1,3A4 | — | — | Y1β, Y3α″ | — | — |
We focused our attention on the specific ISD ions observed from LNDFH-I and LNDFH-II. The goal was to estimate the ratio of the two compounds in mixtures based on the measurement of the relative intensities of specific ISD ions. By varying the mixing ratio of LNDFH-I and LNDFH-II between 0/100, 25/75, 50/50, 75/25, and 100/0 (total glycans concentration is 20 pmol/μL), the intensities of ISD ions at m/z 842.3 (B4), 860.3 (C4), 902.3 (2,4A5), and 962.3 (1,3A5) generated from LNDFH-I gradually increased, whereas those at m/z 511.2 (Y2α) and 568.2 (C3α/Y3α″), which originated from LNDFH-II, decreased. The ion intensities of specific ISD ions at m/z 962.3, 902.3, and 568.2 were normalized to that of the ISD ion at m/z 876.3 which was generated from both LNDFH-I and LNDFH-II. Figure 9 shows the calibration curve and the ratios of the relative intensities of ISD ions as a function of the mixing ratio of LNDFH-I. The calibration curves show a linear relationship and their coefficient values (R2) were over 0.95. Therefore, the molar ratio of LNDFH-I and LNDFH-II in the mixtures could be estimated from the normalized intensities of their specific ISD fragments.

MALDI-ISD and -PSD occur in same experimental conditions, but in different time scales. We next compared MALDI-ISD with MALDI-PSD which involves the fragmentation of the metastable decay of [M+Na]+ occurring in the field-free drift path of a time-of-flight mass spectrometer. The mechanism of PSD can be explained by vibrational activation processes.48) As expected, the MALDI-PSD mainly generated fragment ions that originated from glycosyl bond cleavage and the mass spectra of LNDFH-I and LNDFH-II were similar, with the only difference between the spectra being a fragment ion at m/z 511.2.35) Therefore, MALDI-ISD with 5-NSA is a better method than positive-ion MALDI-PSD for the identification and semi-quantitative analysis of glycan isomers.
Yamagaki and co-workers reported that negative-ion the MALDI-ISD and MALDI-PSD of glycans causes the production of cross-ring fragment ions with a high intensity, which can provide information on the linkage positions.49,50) Negative-ion MALDI-PSD has been previously demonstrated to be useful int the semi-quantitative analysis of same glycans (LNDFH-I and LNDFH-II) at a concentration of 1.5 mg/mL.51) The negative-ion MALDI-PSD spectra of LNDFH-I and LNDFH-II gave specific informative fragment ions and could be used for the semi-quantitative analysis of a mixture of glycan isomers. However, the detection limit of positive-ion MALDI-ISD with 5-NSA was found to be 100 times better than that for negative-ion MALDI-PSD.
Redox reactions of 5-NSA molecule during MALDI-ISD processesAs described above, the use of 5-NSA leads to MALDI-ISD involving “hydrogen-deficient” peptides and glycan radical precursors. This suggests that reductive reactions of 5-NSA molecules occur in the MALDI process. Figure 10 shows positive- and negative-ion MALDI mass spectra of 5-NSA. 5-NSA gave molecular-related signals [M+H]+, [M+Na]+, and [M−H]−. Other product signals of [M–15+H]+, [M–16+H]+, [M–17+H]+, [M–30+H]+, and [M–30+Na]+ in Fig. 10a and [M–16–H]– and [M–30–H]– in Fig. 10b were also observed. It is known that the reduction of a nitro group –NO2 leads to the formation of an amino group –NH2, with intermediate stages that involve the formation of a nitroso group –NO and a hydroxylamine group –NHOH.52) It has also been reported that the nitro group moiety is partially converted into a nitroso group and an amino group by hydrogen transfer reactions during the MALDI process.53,54) The reduction reactions of 5-NSA are shown in Scheme 12. The loss of an oxygen atom from the nitro group of 5-NSA to form the reduced product [M–16] containing a nitroso group –NO is involved in two hydrogen reduction process and is accompanied by the loss of water. The radical species [M–17] is formed by a hydrogen transfer reaction from [M–16] to other 5-NSA molecules. The reduced product [M–30] containing an amino group –NH2 is formed from further reduction reactions of [M–16] via the transfer of four hydrogen atoms. 5-NSA participates in a variety of reductive reactions during the MALDI process. “Hydrogen-deficient” analyte radicals and reduced 5-NSA are formed by the transfer of hydrogen from the analyte to 5-NSA.


The use of an oxidizing matrix, namely 5-NSA, in the MALDI-ISD of peptides promotes fragmentation pathways that involve the abstraction of hydrogen from the peptide backbone. The first step in MALDI-ISD with 5-NSA involves the intermolecular transfer of hydrogen from an amide group in the peptide backbone to 5-NSA, leading to the formation of a “hydrogen-deficient” peptide radical and reduced 5-NSA. This is followed by subsequent radical induced Cα–C bond cleavage, leading to the formation of an a•/x fragment pair. Radical a• fragments undergo further hydrogen abstraction to form a ions after Cα–C bond cleavage. In contrast, the CO–N bond at Xxx–Pro was specifically cleaved via hydrogen abstraction from the Cα–H in Pro residue. CO–N bond cleavage leads to the formation of a b•/y fragment pair and radical b• fragments undergo either further degradation or hydrogen abstraction to form b or a fragments after CO–N bond cleavage.
For the MALDI-ISD analysis of glycans, the use of a conventional reducing matrix does not lead to radical fragmentation of glycans. On the other hand, both glycosidic and cross-ring cleavage is promoted by the abstraction of hydrogen from hydroxyl group of glycans by 5-NSA molecules. MALDI-ISD with 5-NSA and its subsequent pseudo-MS3 are useful methods for identifying glycans, including linkage analysis. Glycan isomers gave specific ISD ions and their intensity ratio depends on mixing ratio of the isomers. The molar ratio of isomers in mixtures can be estimated from the intensities of their specific ISD fragments. MALDI-ISD with 5-NSA could be a useful method for structural analysis of both structural and semi-quantitative analysis of glycans.
Fragmentation that occurs in MALDI-ISD with 5-NSA is mechanistically similar to that by oxidation-based fragmentation techniques, EDD, NECD, and fs-LID. In principle, the applicability of EDD/NECD and fs-LID is limited to the fragmentation of multiply-charged deprotonated molecules and singly-charged protonated molecules, respectively. On the other hand, MALDI-ISD is initiated by a direct hydrogen transfer, so that the ISD fragments are observed in the both positive- and negative-ion mode. Therefore, this will allow MALDI to be used for the structural analysis of both basic and acidic biomolecules.
This article is a summary of one part of my research work which was awarded by the Mass Spectrometry Society of Japan, MSSJ Research Award (June 1st, 2012). Studies of the ion and radical formation mechanisms in electrospray droplet impact/secondary ion mass spectrometry are not included in this article to avoid redundancy. An integrated article of this subject has already been published in J. Mass Spectrom. Soc. Jpn.55)
My research was supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists (DC: 20-11732 and PD: 23-10272).