2013 Volume 2 Issue Special_Issue Pages S0007
2013 Volume 2 Issue Special_Issue Pages S0007
The introduction of DART and DESI sources approximately seven years ago led to the development of a new series of atmospheric pressure ion sources referred to as “ambient ionization” sources. These fall into two major categories: spray techniques like DESI or plasma techniques like DART. The selectivity of “direct ionization,” meaning analysis without chromatography and with little or no sample preparation, depends on the mass spectrometer selectivity. Although high resolution and tandem mass spectrometry are valuable tools, rapid and simple sample preparation methods can improve the utility of ambient ionization methods. The concept of ambient ionization has led to the realization that there are many more ways to form ions than might be expected. An interesting example is the use of a flint-and-steel spark source to generate ions from compounds such as phenolphthalein and Gramicidin S.
The term “ambient ionization” was originally applied to Desorption Electrospray Ionization (DESI)1) and Direct Analysis in Real Time (DART)2) when these techniques were first introduced in late 2004 and early 2005. The term was originally used to describe ion sources that operated in open air with ionization occurring in the laboratory environment instead of within an enclosure or a vacuum chamber. However, “ambient ionization” has come to mean any atmospheric pressure ion source that provides direct analysis with minimal sample preparation.
These terms can be confusing. If the definition of “ambient” is “the surrounding environment,” then the ambient condition of a sample in vacuum is vacuum. “Direct analysis” can have an even broader meaning. For example, a sample introduced into an electron ionization (EI) ion source by a direct insertion probe could be considered “direct ionization.”
If we carry out an analysis of a complex mixture without chromatography or sample cleanup, we must rely on the specificity of the mass spectrometer. In most cases, that means the use of high resolution and exact mass measurements and/or tandem mass spectrometry (MS/MS). However, adjusting the ionization chemistry or using sample preparation or cleanup methods that are rapid and convenient can enhance the value of ambient ionization methods. Although it may seem contradictory, ambient ion sources have also been combined with separation methods including GC, LC, TLC and ion mobility.
Terminology aside, these new ion sources have led to many new developments and applications. In Greek mythology, opening Pandora’s Box resulted in the release of all of the bad things in the world. In contrast, ambient ion sources opened the “box” of the atmospheric pressure ion source, resulting in the release og good things: development of a growing number of new atmospheric pressure ion sources and a rapidly increasing number of acronyms.3–7)
The new atmospheric pressure ion sources fall into two categories: spray techniques like DESI that are related to electrospray ionization (ESI) and plasma techniques like DART that are related to Atmospheric Pressure Chemical Ionization (APCI). Hybrid techniques such as those combining lasers and DESI or DART have also been developed. With apologies to the researchers who have developed all of the new ion sources, the number of references is too large to list here. However, descriptions of the new sources and references can be found in the review articles cited in the previous paragraph.
Our recent work has primarily focused on DART and its applications. Like Atmospheric Pressure Photoionization (APPI), DART is a type of APCI. The principal difference between these methods is the initial ion formation step. In APCI, the initial ionization step is a corona discharge. In APPI, the first step in ion formation is photoionization. In DART, ions are initially formed by Penning ionization. That is, interactions of long-lived excited states (metastable atoms or molecules) with an analyte or atmospheric gases. The reaction sequence that follows depends on not only the initial ion population, but on a series of reactions that occur as ions are transported into the vacuum system.
An example of “pure” ambient ionization is the detection of 2-propenesulfinic acid, a transient highly reactive intermediate that is formed when onions or garlic are bruised. By bruising garlic at room temperature in the DART gas stream, we were able to detect 2-propenesulfinic acid as a deprotonated molecule with a lifetime of approximately one second.8) This experiment is an ideal candidate for ambient ionization because it can only be carried out with a very gentle ionization method and easy access to the ion source.
Another “ideal” application of DART is in screening for illicit drugs.9) We have used accurate mass, isotopic measurements and fragment ions to detect the presence of drugs of abuse including new designer drugs that are becoming a growing threat.10,11) Targeted methods are not applicable to the detection of new and unexpected drugs that are appearing “on the street” every day.
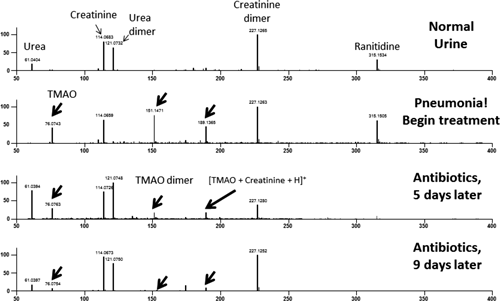
An example where non-targeted screening provides important information was experienced by the author. A case of bacterial pneumonia was treated with azithromycin. Upon analyzing a urine specimen for excreted azithromycin, unexpected peaks were observed that were assigned as trimethylamine N-oxide. A search of the human metabolomics database12) revealed that this was a biomarker for kidney failure. Fortunately, the condition was an acute response to the bacterial infection that improved rapidly upon recovery (Fig. 1).
Rapid and convenient sample preparation methods and in-situ derivatization can be useful tools in conjunction with DART. We have carried out in-situ silylation and methylation to facilitate the detection of thermally labile species such as phosphates or polysaccharides. Sample preconcentration methods such as solid-phase microextraction (SPME) or stor-bar-sorptive extractions (SBSE) have been particularly useful. In two independent studies, SBSE combined with DART achieved part-per-trillion detection limits for contaminants in water.13,14)
Several applications of DART to chemical fingerprinting have been published.15–18) This application relies on chemometrics and does not necessarily require separation or identification of all components. An exciting development is the demonstration of a DART method for ovarian cancer screening that gave results that were statistically 100% accurate.19) The success of this approach may result from the monitoring of a very large number of biomarkers, whereas approaches based on only a few biomarkers can be subject to wide variation.
An interesting observation is that HPLC can be combined with DART, permitting the use of phosphate buffers without ion suppression.20,21) This may be the long-sought-after solution to LC/MS analysis using the same buffer systems that are familiar to chromatographers.
Quantitative analysis is clearly possible with ambient ionization. In the case of DART, the principal obstacle is gas turbulence. Adding an internal standard is an effective solution because the analyte and internal standard will be affected by turbulence in the same way, so their ratios are still quantitative.22–26) Summing three replicate measurements appears to be sufficient to average out the effect of turbulence.27) Transmission DART, in which the gas passes through a screen, minimizes turbulence and improves quantitation.28) A gas transfer tube with vacuum assist also improves the reproducibility of sampling.29) A useful approach combining transmission DART with resistive heating30) and a gas transfer tube is now commercially available from the manufacturer of the DART (Ionsense, Saugus, MA, USA).
Small-molecule H/D exchange by adding D2O to the DART gas stream was used to confirm the presence of melamine in contaminated pet food.31) We have recently combined H/D exchange with collisional activation to obtain structural clues for fragment ions from isomers such as theobromine and theophylline by considering the number of proton exchanges that remain upon fragmentation.
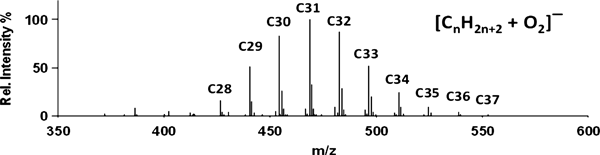
A recent observation is that some nonpolar compounds that are difficult to analyze by DART (including large saturated hydrocarbons and alcohols) can be detected as O2−• adducts by introducing the sample directly into the mass spectrometer atmospheric pressure interface (API) while the DART is operated in negative-ion mode.32) Adduct formation occurs because of the polarizability of the analytes. Supersonic expansion in the No fragmentation is observed, making this a very soft ionization technique for suitable compounds. An example is shown in Fig. 2 for ParafilmⓇ (Pechiney Plastic Packaging Company, Chicago, IL, USA).
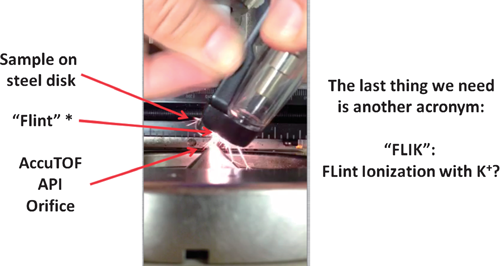
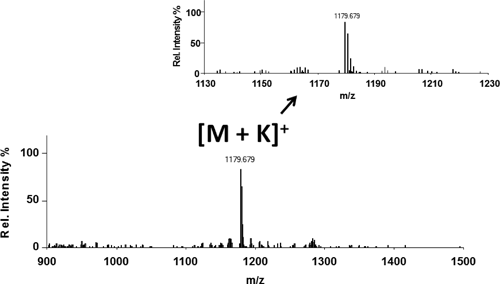
A take-home lesson from ambient ionization is that ion sources can be made in simple and unexpected ways. An amusing example is the use of a flint-and-steel spark source for a butane torch or cigarette lighter to generate potassium adducts from small molecules and peptides. Figure 3 shows a photo of the experiment. The compound to be ionized was deposited on the abrasive wheel that had previously been treated with a KCl solution in methanol. The DART ion source was not installed. Sparks were generated when the wheel was rotated against the “flint.” A transient signal corresponding to [M+K]+ was observed with the time-of-flight mass spectrometer whenever a spark entered the mass spectrometer API interface. Figure 4 shows the mass spectrum for the cyclic peptide Gramicidin S. The ionization mechanism is postulated to be similar to the K+IDS technique.33) Although the practical utility of this method is questionable, it illustrates the point that it is much easier to form ions than one might expect!