2017 Volume 6 Issue 1 Pages A0058
2017 Volume 6 Issue 1 Pages A0058
Collision-induced dissociation (CID) is the most common tool for molecular analysis in mass spectrometry to date. However, there are difficulties associated with many applications because CID does not provide sufficient information to permit details of the molecular structures to be elucidated, including post-translational-modifications in proteomics, as well as isomer differentiation in metabolomics and lipidomics. To face these challenges, we are developing fast electron-based dissociation devices using a novel radio-frequency ion trap (i.e., a branched ion trap). These devices have the ability to perform electron capture dissociation (ECD) on multiply protonated peptide/proteins; in addition, the electron impact excitation of ions from organics (EIEIO) can be also performed on singly charged molecules using such a device. In this article, we review the development of this technology, in particular on how reaction speed for EIEIO analyses on singly charged ions can be improved. We also overview some unique, recently reported applications in both lipidomics and glycoproteomics.
Recent developments of various dissociation techniques have contributed to significant progress in the structural analysis of biomolecules by mass spectrometry. Traditional collision-induced dissociation (CID)1) is categorized by thermal dissociation as well as infrared multiphoton dissociation (IRMPD),2) and some of the weakest bonds in a molecular ion are preferentially cleaved by the excitation of molecular vibrations.3) CID is installed in many commercial mass spectrometers because it can be performed easily in a gas contained reaction cell. However, there are difficulties associated with CID in that the dissociation does not provide unique and unambiguous fragments for molecular structural characterization. In addition to CID, complimentary dissociation techniques, i.e., electron-based dissociation methods,4–6) have been developed over the past decade.7) Electrons are applied to or detached from precursor ions to produce radical states that can be dissociated using an electron beam or an ultraviolet laser beam.8)
The electron impact excitation of ions from organics (EIEIO) was reported by Cody and Freiser in 1979,4) and was the earliest report dealing with the dissociation of singly charged molecular ions by an electron beam in a Fourier transform–ion cyclotron resonance (FT-ICR) mass spectrometer. Analogous to CID, energetic free electrons (∼10 eV) were applied to dissociate small molecules. In 1998, Zubarev, Kelleher, and McLafferty reported on the electron capture dissociation (ECD) of multiply protonated peptides in an FT-ICR mass spectrometer using free electrons with a kinetic energy of nearly-zero.5) In 2004, electron transfer dissociation (ETD), where an anion provided a low energy electron, was reported by Syka, Coon and Hunt in a radio-frequency (RF) quadrupole ion trap.6)
ECD and ETD have both been extensively explored for use in proteomics applications.9–16) Charge-reduced species generated by capturing an electron, which is the intermediate state with an unpaired electron, were dissociated into unique c′ and z· fragments that are cleaved at N–Cα bonds.5,17) Weakly bonded posttranslational-modifications, such as phosphorylation11) and glycosylation,12,13) remain intact on the ECD/ETD fragments, which provide information regarding the location of the sites that are modified on the peptide backbones. ECD also cleaves disulfide bonds, which is unique characteristic that cannot be accomplished using CID.10)
ECD and ETD are similar in their reaction mechanics, but ECD has some practical advantages compared to ETD. In ECD, it is possible to easily control the kinetic energy of electrons at any value (so called hot ECD18)) because free electrons are used for the reaction. The extra energy provided improves the extent of dissociation of intermediate radical states especially for large molecular ions.13,18,19) ETD, on the other hand, is widely used but it often needs additional collisional activation or infrared laser activation before and/or after ETD.19–21) In addition, ETD cannot be applied to singly charged precursor ions because a singly charged positive ion would be lost from the trap by charge neutralization.
The development of ECD in the higher-pressure region within a smaller instrument than the conventional FT-ICR MS is important for use in industry. Although c′ and z· fragments were searched intensively in RF ion trap mass spectrometers, which were gas chromatograph detectors equiped an electron sources for electron impact (EI) ionization, such fragments were not found, because it was too difficult to introduce low energy electrons across the high RF field in the Paul trap.22) This problem was overcome by Baba and coworkers in 2004.23) They introduced low energy electrons along the potential minimum of a linear RF ion trap24) where a static magnetic field was applied (namely a magneto-linear ion trap). Silivra and coworkers reported on the use of ECD in a Paul trap, to which a magnetic field was applied.25) Ding and Brancia demonstrated ECD in their digital ion trap using a digital RF phase at ground voltage.26) An RF-field-free ECD method, which employs a magnetic field to confine electron gas in the ion path, has been under intense development by Voinov and coworkers for the past ten years.27)
The magneto-linear ion trap23,24,28) was recognized as the most efficient ECD device in 2007,29) however, we found further opportunities to improve the performance of this instrument as well as making it easier to use in bioanalytical applications. We developed a new type of RF ion trap for electron based dissociation applications, i.e., a branched ion trap.30) In this review article, we describe improvements to the fast reaction speed. We also describe some unique applications that have recently been explored for use in proteomics and lipidomics. Not only reviewing prior published results but we also show new features that have not been published, including an O-linked glycosylation analysis using LC-ECD-MS and an investigation of the adulteration of extra virgin olive oil using EIEIO.
The origin of the branched ion trap is a branched ion guide that was developed by Thomson and coworkers31) (Fig. 1). It had a four-way-crossed branched structure, which allows multiple ion sources to be connected to a mass spectrometer. The center part of the structure was composed of eight L-shaped electrodes, where an RF voltage was applied (Fig. 2a). This RF field produced six-way-cross pseudo potential minima (dotted lines in Fig. 2b). In the branched ion guide (Fig. 1), two of the branches were used to connect two ion sources, and one was used to couple a mass analyzer through a quadrupole ion guide (Q1). For ion trap operation,30) six planar electrodes, which are biased separately by DC voltages, are placed at each branch (Fig. 2b). Four branches (or two axes) are used for the reacting analyte ions with an electron beam, i.e., an axis (L1–L2) is used for ion injection and ejection, and another axis (L3–L4) is used for electron beam injection. A magnetic field is applied along the electron-beam axis using two neodymium ring magnets (Fig. 2c). The top and bottom branches are blocked by two planar electrodes. The L3 and L4 electrodes are composed of soft iron, and function as a pair of magnetic pole pieces to focus the magnetic field at the center of the trap. The device was placed between the Q1 mass filter and the Q2 collision cell in a time-of-flight mass analyzer (TOF-MS). TOF-MS is advantageous for ECD/EIEIO applications because it has a high signal-to-noise ratio using a multichannel plate (MCP), which results in a high degree of sensitivity. This TOF-MS also has a resolving power of ∼30,000, making it possible to tell a difference in constituents in a number of oxygen, carbon and nitrogen atoms in most lipid ions. Such a high-resolution performance is also useful in proteomics, where highly charged fragment ions are often overlapped in a narrow mass range.


Thermal electrons are generated by electron emitters, which are comprised of a tungsten filament (in earlier versions) or an yttria (Y2O3) coated iridium disk (in current versions). These electrons are extracted by a gate electrode (Gate 1), which has a bias that can be turned on and off, thus permitting the electron beam irradiation to be controlled. Gate 2 was always closed so that the electron beam is kicked back to be trapped in the ECD/EIEIO device. Overall electron capture efficiency was improved by a factor of 2 relative to the case in which Gate 2 was opened. The kinetic energy of the electron beam is controlled by the DC bias of the electron emitter relative to the L-shaped trap electrodes. The actual electron energy should be calibrated by the difference between the work functions of the heated emitter material (∼4.6 eV for tungsten and ∼2.6 eV for Y2O3) and the trap electrode material (stainless steel, ∼4.4 eV). The filament is operated by a DC power supply in the constant voltage mode.
This four-way crossed configuration of the electron and ion axes allows electrons and ions to be controlled independently, i.e., precursor ions are injected into the continuous electron beam (or electron cloud), which is analogous to the beam-type CID in a quadrupole-TOF instrument. We have reported on two types of electron-capture operations: trapping mode and simultaneous ion/electron trapping mode.30) The trapping mode is equivalent to conventional ECD operation, where precursor ions that are isolated by Q1 are introduced into the device during the time when the electron beam is off. After closing the inlet electrode (L1), the electron beam is applied. Product ions and unreacted precursor ions are extracted to the mass analyzer after completion of the reaction. This trapping mode should be selected for fundamental chemistry research because the duration of the reaction is well defined. In the simultaneous trapping mode, on the other hand, electrons are introduced into the device during the ion loading period.30) This mode may be advantageous for highly sensitive applications because it allows ∼100% of the precursor ions to undergo dissociation. After the simultaneous trapping period (typically 10–50 ms), the product ions and remaining precursor ions are extracted to the mass analyzer. In a previous study, we reported that sensitivity is improved by a factor of 2 compared to the trapping mode.30)
Figure 3 shows ECD spectra for three simultaneous trapping periods, which were reported in a previous report.30) Little dissociation was observed under flow-through conditions where the ions were passing through the electron cloud only once (Fig. 3a). Dissociation was dramatically increased when the exit gate (L2) was closed for a few tens of ms (Figs. 3b and 3c). When we closed the gate longer than the case shown in Fig. 3c, however, the total fragment intensity decreased because the fragments were neutralized and the space charge of the trapped ions was saturated (Fig. 4b in ref. 32).

We compared the reaction speed using two grades of magnet materials. One was a neodymium N42 grade (1.21 [T], model RX8CC, K & J Magnetics, Inc., PA, USA), and the other was N52 grade (1.47 [T], K & J Magnetics, Inc.) magnet, which is the strongest permanent magnet commercially available. The ECD spectra of triply protonated neurotensin shown in Fig. 4 were obtained using the two magnetic materials. The filament activation current and the extraction bias between the emitter and the gate were tuned to produce the maximum ECD efficiency. Interestingly, the extraction voltage for the N52 grade was twice as high as that for the N42 magnet. This increase suggests that electrons are less dispersed in the RF field-free region (i.e., inside the electron source unit) by the stronger magnetic field so that a stronger electron beam (or dense electron space charge) could be introduced into the ion trap area. The stronger magnetic field also holds electrons longer in the ion trap area against the ion trap RF field. The overall product intensity was three times higher when the N52 magnets were used.

Barium doped dispenser cathodes, which are often used in FT-ICR MS in ECD experiments,18) needs an ultra high vacuum to operate; thus, this device could not be employed in the pressure region of our electron dissociation device (base pressure of ∼1×10−3 Pa). While a tungsten filament or a thoriated tungsten filament was a conventional choice,23,28) an yttria (Y2O3)-coated iridium disk (Kimball Physics Inc., NH, USA) is currently the best solution for a source of electrons in our applications. Yttria is operable at a lower filament current (∼2.0 A) because its work function is lower (2.6 eV) than tungsten (4.6 eV). It is robust in the medium vacuum pressure range (~ mPa) because it is already oxidized, and the disk produces an electron beam with a larger diameter than the filament.
We compared the performances of the two electron sources, a tungsten filament (ES-032, ETEC compatible model produced by Kimball Physics Inc., active area: 0.05 mm×0.2 mm) and yttria-coated iridium disk (model ES-525, Kimball Physics Inc., active area: 0.84 mm diameter) (Fig. 5). The precursor ions were triply protonated neurotensin, and the operation conditions are shown in Fig. 5. The yttria disk performed ECD better than the tungsten filament in all aspects. Precursor ion consumption was two times higher (diamonds in Fig. 5b vs. Fig. 5a), product ions were two times more abundant (circles in Fig. 5a vs. Fig. 5b), and ion capacity, which is the applicable ion number for an ECD reaction, was three times higher (squares, and maximum positions indicated by arrows in Fig. 5a vs. Fig. 5b). In addition to the ECD performance, it is worth noting that no significant degradation in electron emission performance was found for over the years when the yttria disk was used. The electric resistance of the tungsten filament, on the other hand, increased with increasing time, suggesting that the filament material was evaporating rapidly.

In summary, the reaction speed of the new electron-capture device using the branched ion trap was sufficiently fast to permit the analysis of both singly charged and multiply charged precursor ions.
We previously used digested bovine serum albumin (BSA) in the first study on the use of a branched ion trap device in LC-ECD-MS.30) In the present review, we show an analysis of glycopeptides using hot ECD.13,18) In 1999, ECD was applied to glycopeptides, and a significant difference in dissociation patterns was found compared to CID,12) i.e., CID dissociated glycans but ECD cleaved peptide backbones without the loss of glycans. This result suggests that the two dissociation technologies are complimentary for identifying the structure of a glycopeptide. However, ECD did not provide sufficient energy to cause the electron captured charge-reduced states to dissociate when a precursor ion contains large glycans or a long peptide backbone.20) Hot ECD using electrons with several electron volts18) instead of supplemental excitation by gas-collision or IR laser irradiation is frequently used in ETD experiments21) in order to improve dissociation efficiency.13) In the following application data, O-linked glycopeptides with a long peptide backbone were analyzed using the simultaneous trapping hot ECD mode. A tryptic digest of bovine fetuin was separated by reversed phase online LC, and ECD was operated in the simultaneous trapping mode with a 49 ms injection/reaction duration and a 7 eV electron energy. ECD-TOF spectra were accumulated for 0.5 s/spectrum. The CID measurement was followed by an ECD measurement to survey the glycan structure (data not shown). As shown in Fig. 6, we detected three peptide backbones of N-linked glycopeptides and one of the O-linked glycopeptides, which was consistent with known glycosylation patterns in fetuin. All antennas were sialylated by N-Acetylneuraminic acid (Neu5Ac) except for a glycopeptide (m/z: 991(4+)) with N-glycolylneuraminic acid (Neu5Gc) as an irregular case.

Glycerophospholipids (GPLs) and sphingolipids (SLs) are critical components of many cellular assemblies and are found in many biological pathways. Many classes of GPLs are the main constituents of the double layer of the cell membrane, and the brain is enriched in sphingomyelins (SMs), where they play a crucial role in insulating nerve cell axons.33,34) Accurate molecular structures remain difficult to obtain using conventional MS techniques. Although the head group (phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylglycerol (PG), phosphatidic acid (PA) and sugars such as glucose (Glu) and galactose (Gal)) and primitive structures of the chain (chain length and number of double bonds) in GPLs and SLs can be identified by the conventional CID approach,35) the locations of the double bonds and regioisomerism (assignment of two acyl groups on the glycerol backbone) are difficult to characterize. Arduous and time-consuming procedures are required, and multiple approaches, such as NMR analysis,36) complicated high energy CID or MS(n) operations37) are required whenever a new structural feature is probed.
Our EIEIO approach using an electron beam with a kinetic energy of 10 eV permits near-complete structures of such lipid classes to be determined in a single MS/MS measurement.32,38) Although the precursor ions are singly charged and the reaction rate between the ions and electrons is lower than that for multiply protonated peptides, 60% of the precursor ions were consumed by electron irradiation within 50 ms. About 50% of the consumed precursors produced fragment peaks, and the other 50% were lost from the trap by recombination with electrons. In this review, we discuss some recent progress on structural lipidomics analysis of complex lipids.32,38,39)
Figures 7a, 7c and 8 show EIEIO spectra of an SM and PCs. The head group was identified by the diagnostic peak similar to that for the case of CID (m/z 184 in the case of PC and SM) (Figures 7a and 7c). Two possible backbones were clearly separated by the peaks at m/z 224 and m/z 226 for PCs, and m/z 225 for SMs (Figures 7a, and 7c).32) In the both cases, a series of the chain fragments appeared in the higher m/z regions (Figures 7a, 7c and 8). Regioisomerism in GPLs was identified by the unique C1–C2 cleavage, which is not observed in CID, in the glycerol backbone by EIEIO38) (Fig. 8).


In this analysis, EIEIO produced two fragment peaks at each carbon–carbon single bond in the chain, i.e., a radical fragment and an accompanying hydrogen-lost non-radical fragment.38) The locations of the carbon–carbon double bonds were easily determined by scaling the spaces between the two neighboring radical peaks, i.e., when the space is 14 amu (–CH2–), the covalent bond is a single bond. Because the double bonds are not cleaved extensively in complex lipids, a missing peak with a 26 amu spacing (–CH=CH–) indicates the location of a double bond. We also identified the cis/trans isomerism in a double bond based on the ratio of the intensities of the radical and the non-radical peaks.40) Since these diagnostic rules were simple with little exception, it was easy to develop automatic de novo analysis software for full structural characterization.32,38,39) We demonstrated structural lipidomics using a combination of this EIEIO approach and a pre-separation by a differential mobility spectrometer (DMS).41) Using this procedure, lipids extracted from a heart,32) a brain,42) an egg38) and other tissues were successfully analyzed.
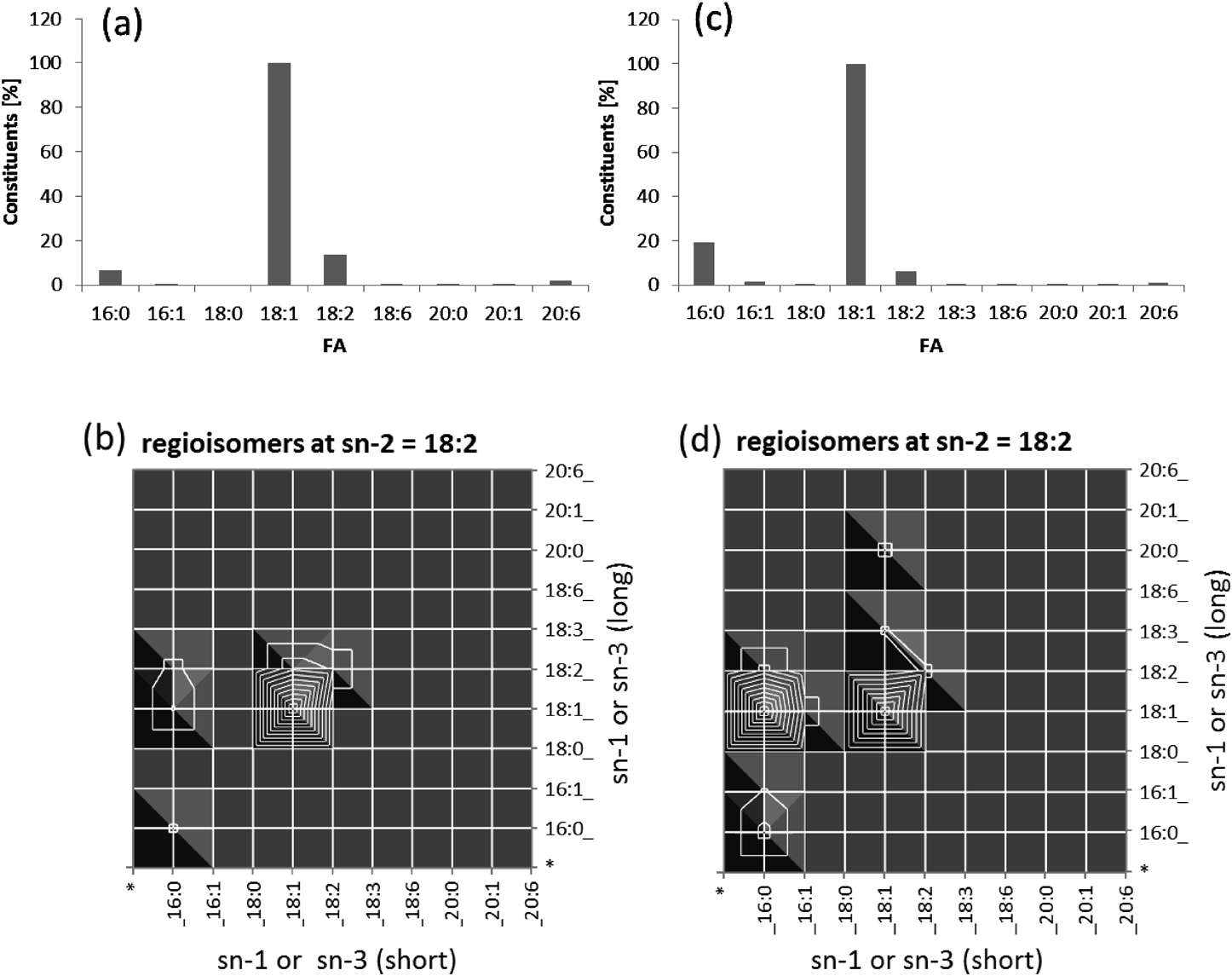
Adulteration of olive oil by hazelnut oilTriacylglycerol (TG) is the most common form of complex lipid used as an energy source and reservoir in plant seeds and animal bodies. One of the current issues regarding TG analysis in food science is the quality inspection of extra virgin olive oil because 70% of “extra virgin” products are estimated to be fake or adulterated. A conventional verification method involves the measurement of the hydrolyzed free fatty acid constituents using GC-MS or LC-MS.43) CID on intact TGs may be helpful in reducing the hydrolysis process, but it still provides only the ratio of the constituent fatty acids.44) We found that EIEIO can provide in-depth information concerning molecular structure, i.e., the structure of the acyl groups and regioisomerism, i.e., whether the acyl groups are located at the center site (sn-2) or outer sites (sn-1 or sn-3) of glycerol.39) It is hard to identify the chirality at C2 in the glycerol backbone by the EIEIO approach at this time, i.e., the specific assignment of an acyl group at sn-1 or sn-3.45) We characterized the regioisomerism of olive and avocado in a previous article.39) A more realistic example, i.e., characterization of the difference between olive oil and hazelnut oil, which is the major adulterant in fake extra virgin olive oil to date, is presented as follows. As a confident standard of extra virgin olive oil, Portuguese ultra- premium extra virgin olive oil was purchased from a special olive oil vendor in Toronto. To obtain raw hazelnut oil, we extracted the total lipids from raw hazelnuts. The detailed procedure of measuring the TG was described previously.39) Figure 9 shows a comparison between olive and hazelnut oils. Although the FA constituents were very similar with each other (Figs. 9a and 9c), EIEIO displayed a significantly different intensity of a TG species, TG-palmitic(C16:0)/linoleic(C18:2)/oleic(C18:1). The relative abundance of this species compared to TG-oleic/linoleic/oleic was much stronger in olive oil than in hazelnut oil. This strategy will be explored further to establish a confident quality control method for verifying real extra virgin olive oil.
We report on the development of an electron based dissociation device using a new type of branched RF ion trap. The velocity of the reaction for the device reached a level that caused the dissociation of singly charged ions when the strongest permanent magnet material and an yttria-coated electron source were used. The device represents a powerful tool not only for conventional ECD applications in proteomics but also for novel applications in structural lipidomics.46)
Mass Spectrometry Society of Japan bestowed the MSSJ AWARD for Technical Development 2017 on the first author. This is an invited review of the achievement.
The authors wish to thank Dr. Eva Duchoslav, Dr. Michael Guna, Dr. Bradley Schneider and Dr. Andre Schreiber for the valuable discussion, Pablo Dominguez, Kenji Yamada, Mircea Toca, Matthew Romaschin and Tiberiu Gera for their engineering support, and Deolinda Fernandes for her excellent work in sample preparation.