2017 Volume 6 Issue 2 Pages S0065
2017 Volume 6 Issue 2 Pages S0065
Flame-induced atmospheric pressure chemical ionization (FAPCI) is a solvent and high voltage-free APCI technique. It uses a flame to produce charged species that reacts with analytes for ionization, and generates intact molecular ions from organic compounds with minimal fragmentation. In this study, desorption FAPCI/MS was developed to rapidly characterize thermally stable organic compounds in liquid, cream, and solid states. Liquid samples were introduced into the ion source through a heated nebulizer, and the analytes formed in the heated nebulizer reacted with charged species in the source. For cream and solid sample analysis, the samples were positioned near the flame of the FAPCI source for thermal desorption and ionization. This approach provided a useful method to directly characterize samples with minimal pretreatment. Standards and real-world samples, such as drug tablets, ointment, and toy were analyzed to demonstrate the capability of desorption FAPCI/MS for rapid organic compound analysis.
The ionization mechanisms of atmospheric pressure chemical ionization (APCI) mainly attribute to a series of ion–molecule reactions (IMRs) between analytes and primary ions.1–6) The proton or charge in the primary ion are transferred to the analyte to form analyte ion.1–3) The metastable atom or molecule, such as He* and N2*, can react with the analytes through Penning ionization processes to form radical cation of analyte.4–6) To generate primary ions and metastable species for APCI processes, several approaches have been developed.1,7–14) The electric discharge-based technique is the most used APCI method, where various reactive species are generated in an electric discharging region under ambient conditions.1,8–12) In a corona discharge ionization source, a high DC voltage is applied to a stainless steel needle to induce electric discharge at the needle tip. The molecules near the discharging region are ionized to form reactive primary ions such as N2+, N4+, NO+, O2+, and H3O+.1,2,15,16) Similar to corona discharge ionization, a DC high voltage is applied to a pin electrode in a direct analysis in real time (DART) or atmospheric pressure glow discharge (APGD) source to induce discharge on the helium gas flowing between the pin and plate electrode.9,17–19) Metastables such as helium (He*) and primary charged species (e.g., NO+, O2+, and H3O+) are generated and used to ionize analytes. In a dielectric barrier discharge (DBD) source, an AC high voltage is utilized to induce a stable electric discharge on helium, nitrogen, or the air flowing between the needle electrode and the dielectric barrier layer (e.g., glass).11,12,20–22) Analytes on sample surfaces are ionized by directing the plasma generated by DBD toward the sample surface.
In addition to the aforementioned APCI sources, other nonelectric discharge techniques capable of generating reactive primary species also ionize analytes via IMRs. Dopant-assisted atmospheric pressure photoionization (APPI) utilizes a Kr lamp (10 eV) to generate primary ions from reagent gases such as toluene, acetone and benzene. The analytes are ionized via charge exchange and proton transfer reactions with the primary ions.23–25) Since an electrospray ionization (ESI) plume contains numerous charged species (e.g., charged solvent droplets, solvent ions, solvent cluster ions, H3O+, and H+), analytes are ionized through IMRs with these charged species in the ESI plume. Laser desorption, pyrolysis, nebulization, and thermal desorption are used to generate analytes from solid or liquid samples.26–35)
A flame produces high temperatures during combustion and has been employed as a tool for atomization for spectroscopic detection of inorganic compounds.36–39) Organic compounds are inevitably decomposed as they are introduced into a flame, therefore, a flame ionization detector (FID) used in a gas chromatography can only measure the ion currents of thermally decomposed products of analytes instead of intact molecule ion signals.40–42) However, previous studies have indicated that numerous reactive primary ion species are generated in a flame; they are useful to ionize analytes through IMRs. A flame-based APCI technique (FAPCI) was developed to generate intact analyte ions.13,14) Rather than using hydrogen gas as the fuel in a FID, carbon-rich fuels such as butane, acetylene, or methanol are burned in a FAPCI source to generate alkyl and formylium ions (CnHm+ and CHO+). The ions are further used to react with water molecules in the air to form H3O+ and (H2O)nH3O+. The charged species are then delivered to the ionization region to react with the analytes generated by nebulizing the sample solutions. Since the analytes are not introduced into a flame and the ionization region is 2–3 cm away from the flame, the analytes are not thermally decomposed and intact analyte ions are produced. In addition, alkali chloride solution can easily be introduced into a flame through a metallic loop to generate alkali ions to react with the analytes with strong alkali affinities. Moreover, the reactive charged species generated by a flame can be directed toward the sample solutions applied on a plate for analyte desorption and ionization.
In this study, desorption FAPCI/MS was applied to directly characterize volatile organic compounds in samples of liquid, cream, and solid states. Liquid samples were introduced into the ionization region through nebulization. Cream and solid samples such as ointments and drug tablets were positioned in front of a flame for thermal desorption and ionization of active ingredients in the samples. Alkali chloride solution was introduced into a flame in a FAPCI to generate alkali adducted sucrose ions. The products of different alkali-sucrose ions were then studied using tandem mass spectrometry.
Chalcone was obtained from Chem Service (PA, USA). Lidocaine, ferrocene, flunitrazepam (FM2), amphetamine (AM), methamphetamine (MA), 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxymethamphetamine (MDMA), sucrose, lithium chloride (LiCl), and rubidium chloride (RbCl) were obtained from Sigma-Aldrich (MO, USA). Sodium chloride (NaCl) was purchased from J. T. Baker (Phillipsburg, NJ, USA). Potassium chloride (KCl) was purchased from Shimakyu’s Pure Chemicals (Osaka, Japan). Methanol (LC grade) and cesium chloride (CsCl) were obtained from Merck (Darmstadt, Germany). Drug tablets and ointments were purchased from local pharmacies. Fine-cut tobacco and toy were obtained from supermarket. Deionized water generated by PURELAB Pulse (ELGA LabWater, Marlow, UK) was used to prepare the sample solutions.
Flame-induced atmospheric pressure chemical ionization sourceThe FAPCI setup has been described in previous publications.13,14) As shown in Fig. 1a, the FAPCI source comprised of a heated oven, nebulizer, and flame. This source was easily constructed by modifying a commercial corona discharge-APCI source (Bruker Daltonics, MA, USA). The corona discharge needle in the commercial APCI source was replaced with a combustion head (0.4 mm i.d.), which was positioned 1.5–2 cm in front of the sampling orifice of the mass analyzer. A mixture of acetylene and oxygen gas was burned at the combustion head and the size of the flame was adjusted by a gas flow meter (Dwyer, IN, USA). The flow rate of acetylene and oxygen was set at 10 and 20 mL/min, respectively, to generate a stable oxyacetylene flame (0.7 cm long). The charged species generated in the flame moved forward to react with the analytes. During FAPCI/MS analysis, the sample solution was delivered in a nebulizer by a capillary with a flow rate of 2.5 μL/min. A nitrogen gas stream (25–30 psi) was applied in the nebulizer to generate fine droplets from the sample solution flowing out of the capillary, which were further vaporized as they passed through a heated tube set at 350°C. The gaseous analytes moved downward to the ionization region, where they reacted with charged species from a flame. An ion trap mass analyzer (Esquire 6000, Bruker Daltonics, Billerica, MA, USA) was used to characterize analyte ions. The mass spectra were recorded at a scan rate of 5 Hz with mass range from m/z 50 to 600. To generate alkali ions for FAPCI ionization, the saturated alkali chloride solution (1 μL) was applied in a small loop on a stainless steel needle. After drying, the needle was inserted into the oxyacetylene flame and the resulting alkali ions were directed to the ionization region for analyte ionization. The Ampl value of the ion trap was set between 0.35 and 1 V to perform MS/MS analysis.
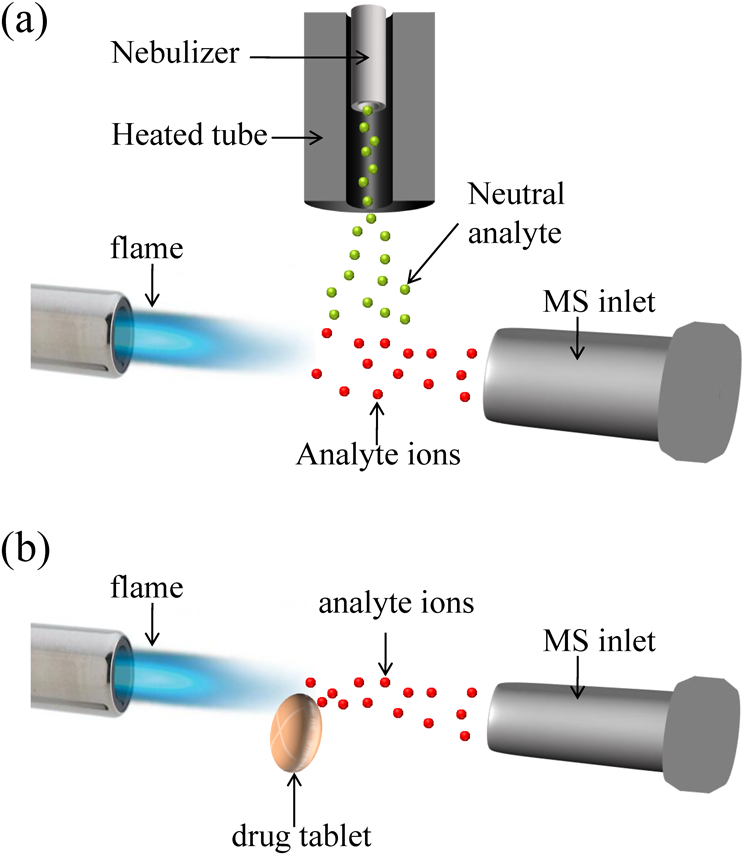
Since numerous charged species are produced in a flame, FAPCI is useful for directly desorbing and ionizing analytes without tedious sample pretreatment. Figure 1b is the schematic illustration of desorption FAPCI/MS (DFAPCI/MS) for characterizing active ingredients in a drug tablet. An oxyacetylene flame was positioned 2.5–3 cm in front of the inlet of a mass analyzer. The acetylene and oxygen flows were set at 10 and 15 mL/min, respectively, with the sample positioned 1.5 cm in front of the flame tip. The analyte ions were characterized using either a quadrupole time-of-flight mass spectrometer (micrOTOF-QII, Bruker Daltonics, MA, USA) or ion trap MS. The mass spectra of Q-TOF were recorded at a scan rate of 1 Hz with mass range from m/z 100 to 450. To characterize liquid and semisolid samples, the sample was deposited on a glass rod and then positioned in front of the flame for DFAPCI/MS analysis. The temperature at the ionization region was determined by a thermocouple (HI 93530, Hanna Instruments, RI, USA) to be 200±25°C.
Standard solutions of chalcone (10 ppm in MeOH) and flunitrazepam (1 ppm in MeOH) were analyzed to test FAPCI’s capability to generate intact analyte ions from organic compounds. A heated nebulizer was used to vaporize the standard solution. The nebulizing gas carried analytes to the ionization region of an FAPCI source to react with flame-induced primary charged species (e.g., CHO+, CnHm+, H3O+, etc.).41,43) Figure 2 shows the background ion mass spectrum of an FAPCI source. Since the ion trap analyzer provided poor ion response at low mass range (m/z <50), HCO+ at m/z 29 and H3O+ at m/z 19 were not detected. Nevertheless, water cluster ions at m/z 55 [H2O]3H+ and 73 [H2O]4H+ were characterized. The ions at m/z 43 [C2H3O+] and a series of ions with 14 mass unit difference (i.e., m/z 57 and 71) were also detected. Since the ionization region was ca. 3.5–4 cm away from the acetylene flame, the temperature in the ionization source was ca. 200°C. To prevent the analytes from thermally decomposing in the source, the time for the analytes to remain in the source was controlled by adjusting the flow rate of nebulizing nitrogen gas. The pressure of the gas was adjusted to 25–30 psi to obtain the highest analyte ion signals. Figure 3 shows the FAPCI mass spectrum of chalcone and flunitrazepam, wherein protonated chalcone [M+H]+ at m/z 209 and flunitrazepam ions [M+H]+ at m/z 314 were successfully detected, while the thermal decomposition products of analytes were not detected at all. It was found that the detection limits for chalcone and flunitrazepam were 5 and 20 ppb, respectively, using an FAPCI or commercial APCI source, where the ion trap analyzer was operated in full-scan mode. Analyte ions were generated through proton transfer reaction between analytes and charged species, indicating that FAPCI is a soft ionization technique to characterize organic compounds.


Since high temperatures induced by a flame can breakdown ionic bonds, the solution containing saturated alkali chloride was introduced into the oxyacetylene flame to produce alkali cations to react with analytes. Figure 4 shows the FAPCI mass spectra of sucrose reacted with different type of alkali ions including Li+, Na+, K+, Rb+, and Cs+, where sucrose was prepared in MeOH with the concentration level of 50 ppm. Without introducing any alkali chloride into the flame, protonated [M+H]+ at m/z 343, dehydrated [M−H2O+H]+ at m/z 325, and ammonium-adducted [M+NH4]+ at m/z 360 sucrose ions were detected by FAPCI/MS (Fig. 4a). The inset in Fig. 4a shows the photograph of an oxyacetylene flame, where a light blue flame is seen at the combustion head. As the solution containing different alkali chloride was introduced into the flame, the flame color changed to red (LiCl), yellow (NaCl), and violet (KCl, RbCl, and CsCl), respectively (see the insets in Figs. 4b–f). The FAPCI mass spectra showed that different alkali-adducted sucrose ions [m/z 349 for (M+Li)+, m/z 365 for (M+Na)+, m/z 381 for (M+K)+, m/z 427 for (M+Rb)+, m/z 475 for (M+Cs)+] were detected as each alkali chloride solution was introduced into the flame (Figs. 4b–f). The protonated, dehydrated, and ammonium-adducted sucrose ions were not detected. The results suggest that sucrose has stronger affinity to alkali ions than H+ does.

To study the attachment of different alkali ions to sucrose, alkali chloride solutions of different concentrations (from 10−6 to 0.5 M) was introduced into the flame. It was found that (M+Li)+ and (M+Na)+ ions were not detected (10−6 to 10−4 M) until the concentration of alkali chloride reached 10−3 M. However, the peak areas of (M+Li)+ and (M+Na)+ increased with the alkali chloride concentration. For other alkali cations (i.e., K+, Rb+, and Cs+), the sucrose-alkali ions were detected as the concentration of alkali chloride solutions was 10−5 M. The order of the lattice energies of alkali chloride was as follows: CsCl (659 kJ/mol)<RbCl (689 kJ/mol)<KCl (715 kJ/mol)<NaCl (787 kJ/mol)<LiCl (853 kJ/mol). The order indicated the formation of Cs+, Rb+, and K+ ions was easier than the formation of Na+ and Li+ ions in the flame. This explained why (Cs-sucrose)+, (Rb-sucrose)+, and (K-sucrose)+ were detected at lower alkali chloride concentrations than those of NaCl and LiCl.
Sucrose is a disaccharide sugar containing a glucose and fructose unit. The MS/MS spectra of protonated sucrose [M+H]+ at m/z 343 showed a series of fragmented ions consisting of dehydrated sucrose (m/z 270 to 330) and dehydrated monosaccharide (m/z 125 to 170) (Fig. 5a). Since the relative molecular masses of glucose and fructose are both 180 g/mol, it is difficult to tell if the dehydrated monosaccharide ions originated from glucose or fructose. Figures 5b–d show the MS/MS spectra of Li-, Na-, and K-sucrose ions. Glucose and fructose ions [i.e., (M(mono)+alkali)+ and (M(mono)+alkali-H2O)+, M(mono)=glucose or fructose] were detected as the prominent fragment ions. However, no fragment ions were detected for (sucrose+Rb)+ and (sucrose+Cs)+ ions (Figs. 5e and f). The exception were Cs+ ions, which were detected on the MS/MS spectrum of (sucrose-Cs)+. The results indicated the attachment of Rb+ and Cs+ to sucrose decreases with the tendency of the ions to fragment. It was supposed that the increase of ionic radius and decrease of electronegativity for Rb+ and Cs+ leaded to a weak metal–sucrose interaction. So that Cs+ ions were detected during MS/MS analysis instead of sucrose fragment ions.

The high temperatures of a flame can also be used to evaporate analytes in samples. This could be done by directing the flame to the sample. The technique, desorption-FAPCI (DFAPCI), is capable of simultaneously desorbing and ionizing analytes in the open air. To characterize analytes in liquid samples, the sample solution (ca. 2 μL) was deposited on a glass rod. The glass rod was positioned near the oxyacetylene flame for analysis. After the analytes were desorbed by the flame’s high temperature, they were subsequently reacted with the charged species in the flame to form analyte ions. Ferrocene is a nonpolar organometallic compound consisting of two cyclopentadienyl rings; its radical cations (M·+ at m/z 186) were detected by positioning a glass rod with dry ferrocene near the flame (Fig. 6a). To analyze polar compounds, protonated lidocaine ion signals ([M+H]+ at m/z 235) were detected on the FAPCI mass spectrum (Fig. 6b) as the glass rod with the sample was moved near the flame. For analysis of mixtures, a solution containing amphetamine, methamphetamine, MDA, and MDMA (i.e., 200 pg each) was deposited on the glass rod for DFAPCI analysis. Protonated amphetamine ([M+H]+ at m/z 136), methamphetamine ([M+H]+ at m/z 150), MDA ([M+H]+ at m/z 180), and MDMA ([M+H]+ at m/z 194) ions were all detected (Fig. 6c).

The setup of DFAPCI is extremely simple, with no high voltages or solvents required. After combining DFAPCI with MS, the technique is suitable to rapidly analyze real-world samples such as over-the-counter drug tablets and ointments. Active ingredients in drug tablets were rapidly characterized by DFAPCI/MS after positioning the tablet near the flame and MS inlet (Fig. 1b). Figure 7a shows the DFAPCI mass spectrum of the COSTI tablet, in which the active ingredient (domperidone, [M+H]+ at m/z 426) was detected. Acetaminophen ([M+H]+ at m/z 152) and a volatile component (caffeine, [M+H]+ at m/z 195) were both detected from a Panadol tablet (Fig. 7b), while propranolol ions ([M+H]+ at m/z 260) were detected from the Propranolol tablet (Fig. 7c). The ointments are generally semisolid substances containing active ingredients and excipients such as propylene glycol, dimethicone, hyaluronic acid, and hydrogenated polyisobutene. To analyze ointments, a glass rod was used to gently scrape ointment surfaces with the rod, it was then placed near an oxyacetylene flame for DFAPCI/MS analysis. It was found that ion signals of active ingredients were predominant on the FAPCI mass spectra; excipients were undetected, likely due to their lower volatilities and ionization efficiencies than those of the active ingredients. Figure 7d shows the DFAPCI mass spectrum of crotamiton ([M+H]+ at m/z 204), which was characterized from the Winsolve Bumin ointment. Diphenhydramine ([M+H]+ at m/z 256) was detected from the Thiam ointment, and Econazole ([M+H]+ at m/z 381 and 383) was detected from the Ledernin ointment (Figs. 7e and f).

The use of DFAPCI/MS for rapidly characterizing chemical compounds on sample surfaces was further explored by using a glass rod to sample the analytes on surface. The sample limitations due to overlarge sizes, irregular shapes, immovable, or inaccessible locations can be avoided by using glass rod for sampling. In this study, the chemical compounds on object surfaces were sampled by a glass rod for DFAPCI/MS analysis. As shown in Fig. 8c, plasticizer ion signals such as bis(2-ethylhexyl)phthalate (DEHP) at m/z 391 [M+H]+ and diisononyl phthalate (DINP) at m/z 419 [M+H]+ were detected on the surface of a plastic toy. Figures 8a and b show the extracted ion chromatogram (EIC) of DEHP and DINP. The fine-cut tobacco was also touched with a glass rod for DFAPCI/MS analysis, which showed strong nicotineions ([M+H]+ at m/z 163) on the mass spectrum (data not shown). The experimental results indicate DFAPCI/MS has the potential to rapidly characterize trace chemical compounds on various objects to assist forensic science and homeland security investigations.

Desorption FAPCI is a simple but versatile ionization technique for generating intact molecular ions of polar or less polar chemical compounds. This novel technique has been utilized to characterize standard compounds and real-world samples. Compared to current atmospheric pressure ionization (API) sources, desorption FAPCI has several unique features and advantages: (i) high DC or AC voltages are unnecessary for ionization; (ii) solvents are not required for ionization, (iii) the construction of the ion source and its operation are extremely simple; (iv) the ion source is maintenance free; (v) different types of analyte-alkali complex ions are easily generated; and (vi) analytes can be directly desorbed and ionized from surfaces. These features make desorption FAPCI not only suitable for routine analysis, but also to serve as an ionization source for in-field instruments like portable or mobile mass spectrometers.
This study was partially supported by Grants provided by the Ministry of Science and Technology of Taiwan.