Abstract
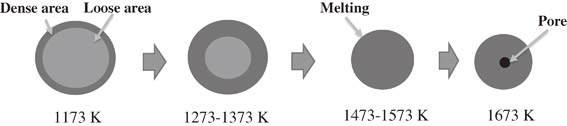
In order to investigate the effect of bio-coal on the carbothermic reduction of laterite ores, carbothermic reduction experiments of two type laterite ores mixed with bio-coal were conducted in this research. The reduction experiments were carried out at 1173–1673 K for 1800 s in Ar. Compared reduction results of coal and bio-coal show increasing the nickel content with increasing temperature. Bio-coal had become the reductant, the nickel content of limonite specimens and saprolite specimens had been raised from 1.18 mass% (before reduction) to 1.64 mass% (after reduction at 1373 K for 1800 s) and from 3.43 mass% (before reduction) to 4.93 mass% (after reduction at 1473 K for 1800 s), respectively. The major phases of limonite specimens were Fe1.833(OH)0.5O2.5 and Fe2SiO2. After the reduction at 1473 K for 1800 s, the major phase was metal Fe. The major phases of the saprolite specimens were Fe(CO3), Fe3O4, Fe2SiO4 and Al3S4. After the reduction at 1473 K for 1800 s, the major phases were Fe, Fe0.64Ni0.36 and α-Fe10.8Ni. The morphological observation of limonite and saprolite specimens shows that the pellet-shaped specimen became a dense area and the core of the pellet had loose structure at 1173–1374 K for 1800 s. The core of pellet was produced small spherical metal phase at 1473 K for 1800 s, this temperature was the soft melting temperature. Finally, the pellet produced nickel-iron alloy phase and iron was separated from the oxide phase at 1673°C for 1800 s.