2016 Volume 57 Issue 10 Pages 1832-1836
2016 Volume 57 Issue 10 Pages 1832-1836
A new magnetic compound in the Mn–Li–N system was synthesized at GPa-order pressure. This compound had a body-centered-tetragonal (bct) structure (space group: I4/mmm, No. 139) with lattice parameters a = 0.29186 nm and c = 0.39108 nm. The compound composition ratio was Mn:N = 2:1. The saturation magnetization and coercivity of a sample synthesized under 5 GPa at 1100℃ for 2 h were 17.73 A∙m2/kg and 310.7 kA/m, respectively.
Mn-based magnetic compounds have attracted considerable attention as a result of their high coercivity, and because they are candidates for a variety of applications. For example, MnBi1) and Mn–Al–C2) are candidates for alternative permanent-magnet materials without critical elements, while Mn3Ga3,4) and Mn3Ge5) are expected to be suitable for spin-torque transfer applications. Some compounds in the Mn–Pnictogen (Pn) system, such as MnBi, have relatively high coercive forces. Furthermoe the amplitudes of the magnetic moments in some Mn–Pn compounds are more than 3.5 μB/Mn atom6) (μB is the Bohr magneton). Therefore, Mn compounds have considerable potential for application in high-performance permanent magnets, and Mn is regarded as a substance of interest in the search for new magnetic materials with high coercivity.
The exploration of novel magnetic compounds in an alkali metal (AM) system was focused in this study. AMs react only minimally with 3d-transition metals (3d-TMs)7) and, as a result, permanent magnet alloys consisting of AMs have been only minimally reported. AMs tend to be sensitive to pressure; for example, the atomic compressibilities of these metals are significantly higher than those of transition metals8). This difference in compressibility causes a change in the relative radii of the atoms and induces atomic rearrangement and high-pressure phases. In fact, many high-pressure phases comprised of AMs have been found. In this study, Li was selected as the AM, because of its low atomic weight, so as to prevent this component from weakening the gravimetric magnetization.
In addition, N, which is one of the Pn elements, was selected as the third element (with Li and Mn). Certain Mn–N binary system compounds, i.e., Mn4N, Mn3N2, Mn2N1-x, and MnN, have been reported previously9). The crystal structure of Mn2N1-x is hexagonal, and differs from those of the other Mn–N compounds. However, the Mn4N, Mn3N2, and MnN crystals structures are similar to each other, having only small differences in their lattice constants, N content values, and N locations. On the other hand, only Mn4N exhibits ferrimagnetism10), while the other Mn–N compounds are non-magnetic at room temperature11–13). Therefore, the magnetism in the Mn–N system is sensitive to either the lattice constants or the N content.
High-pressure synthesis is also employed in this study. This is an effective method for exploring novel compounds and materials, in which samples are heat-treated under GPa-order pressure. Many new compounds have been identified using this technique, for example, in the fields of hydrogen storage materials, superconducting materials, and thermoelectric materials14–16). However, this method has not been applied to the search for novel magnetic materials.
The purpose of this study is to explore a new compound in the Mn–Li–N system using high-pressure synthesis, and to investigate the crystal structure and magnetic properties of the newly found compound.
Mn (99.99-mass% purity) and LiNH2 (95-mass% purity) powders were used as starting materials. LiNH2 was used as a source of both Li and N, because this material has significantly higher N content than other Li–N based compounds. Nominal-composition powder mixtures were pressed into pellets and sealed into a high-pressure sample cell. In this cell, the samples were packed by sintered hexagonal boron nitride (h–BN), which acted as the pressure medium. High-pressure synthesis was then conducted using a multi-cubic anvil-type apparatus. The sample cells were heated at 1100℃ for 2 h under pressures of 2–6 GPa and then quenched. Note that the excess hydrogen exited through the sample cell during the synthesis reaction.
Phase identification was conducted via powder X–ray diffraction (XRD) analysis using Cu–Kα radiation. Compositional analysis was performed using an electron probe micro-analyzer (EPMA) or through scanning electron microscope/energy -dispersive X–ray analysis (SEM–EDX). The magnetic properties were investigated using a vibrating sample magnetometer (VSM). Some samples for microstructural observation were prepared using a focused ion beam (FIB) system.
Figure 1 shows the XRD patterns of the Mn+LiNH2 samples treated at 1100℃ for 2 h under pressures of 2–6 GPa. The molar ratio of the Mn:LiNH2 nominal composition was 1:1. Unidentified peaks were observed for all of the samples, and ζ–phase (Mn2N1-x) was observed in the samples subjected to pressures of 2–4 GPa. The intensities of the unidentified peaks increased with increasing applied pressure. In the sample prepared under 5 GPa pressure (hereafter, the “5-GPa sample”), the unidentified peaks were mainly observed with small h–BN and cubic–BN (c–BN) peaks, corresponding to a diamond-type crystal structure. The unidentified peaks weakened when the applied pressure was increased to 6 GPa; however, the c–BN peak intensities increased. Under high-pressure and high-temperature conditions, Li-based compounds such as LiNH2 are reported to act as catalysts to transform h–BN into c–BN17). Thus, it was assumed that the h–BN sealed in the high-pressure sample cell as a pressure medium transformed into c–BN, because of the presence of LiNH2 in the samples.
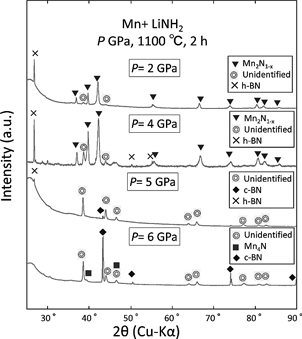
XRD patterns of Mn+LiNH2 samples treated at 1100℃ for 2 h under pressure P = 2–6.
Figure 2 shows the (a) coercivities and (b) saturation magnetization of the Mn+LiNH2 samples treated at 1100℃ for 2 h under 2–6 GPa. Although some discrepancies are apparent in the overall trends, both the coercivity (310.7 kA/m) and saturation magnetization (17.7 A∙m2/kg) of the 5-GPa sample, which was primarily comprised of the unidentified phase according to the XRD results, were higher than those of the other samples. Figure 3 shows the magnetization curve measured by VSM of the 5-GPa sample. Based on the XRD and VSM results, the unidentified phase was assumed to have high coercivity.
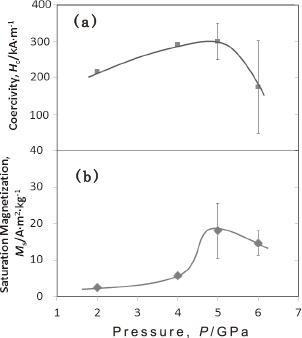
Magnetic properties of Mn+LiNH2 samples treated at 1100℃ for 2 h under P = 2–6 GPa: (a) coercivity and (b) saturation magnetization.

Magnetization curve of Mn+LiNH2 sample treated at 1100℃ for 2 h under P = 5 GPa.
To determine the features of the unidentified phase, a composition analysis was conducted. Figure 4 shows (a) a back-scattered electron (BSE) image and (b)–(d) Mn, N, and B element mappings (EPMA), respectively, for the Mn+LiNH2 sample treated at 1100℃ for 2 h under 5 GPa. The dark phase in the BSE image is assumed to be due to BN dispersed by the FIB process during the sample fabrication. The composition ratio of the primary light phase was estimated to be Mn:N = 2:1 from chemical composition analysis. B was not observed. Apart from BN, the only unidentified phase apparent in this image corresponds to that observed in the XRD results for this sample (Fig. 1). Therefore, the primary light phase can be taken as the unidentified phase, having a composition ratio of Mn:N = 2:1. Note, however, that EPMA analysis is unable to detect the presence of Li, as this is a very light element.
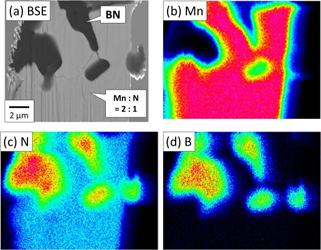
EPMA images of Mn+LiNH2 treated at 1100℃ for 2 h under P = 5 GPa. (a) Back-scattered electron (BSE) image and element mappings of (b) Mn, (c) N, and (d) B.
Therefore, an Mn–N binary sample without Li was also synthesized, so as to confirm the presence of Li in the unidentified phase in a paradoxical manner. Figure 5 shows the XRD patterns of Mn2N samples before and after high-pressure heat treatment at 1100℃ for 2 h under 6 GPa. After this treatment, the Mn2N1-x peaks remained and the unidentified phase was not observed; this was despite the fact that the Mn:N composition ratio in the sample was identical to that of the unidentified phase, i.e., Mn:N = 2:1. As the unidentified phase was not synthesized without Li, this element was therefore assumed to play a role in the formation of the unidentified phase.

XRD patterns of Mn2N samples before and after high-pressure heat treatment at 1100℃ for 2 h under P = 6 GPa.
The crystal structure of the unidentified phase in the Mn–Li–N system was then investigated. However, it was difficult to determine the Li content or position, because of this element's small atomic number. Therefore, the crystal structure was examined in the context of the Mn and N sublattices only. A body-centered-tetragonal (bct) structure (space group: I4/mmm, No. 139) was suggested. Figure 6 shows the XRD patterns of both the 5-GPa sample and a simulated bct structure. The lattice constants of the bct structure were determined to be a = 0.29186 nm and c = 0.39108 nm, and the simulated pattern exhibited good agreement with that of the 5-GPa sample. The atomic positions and crystal structure of the bct structure are shown in Table 1 and Fig. 7, respectively. The N occupancy was estimated by considering a composition ratio of Mn:N = 2:1. Note that Enoki and Ohtani18) have estimated the crystal structure and magnetic properties of the bct compound using ab–initio calculations. Good agreement is obtained between the experimental results of the present study and the values calculated by Enoki and Ohtani18), when the N-site vacancies are located in the center of c-plane. They also suggested that some Li-atoms might be substituted a part of Mn-sites.
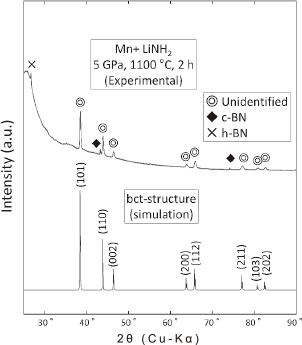
XRD patterns of Mn+LiNH2 treated at 1100℃ for 2 h under P = 5 GPa and of simulated bct structure.
| Space group: I4/mmm (No. 139) a = 0.29186 nm c = 0.39108 nm |
||||
|---|---|---|---|---|
| x | y | z | Atom | Occupancy |
| 0.0 | 0.0 | 0.0 | Mn | 1.0 |
| 0.0 | 0.0 | 0.5 | N Vacancy |
0.5 0.5 |

Suggested crystal structure of Mn–Li–N bct phase.
The composition ratio of the new bct phase, Mn:N = 2:1, is similar to that of the ζ–phase (Mn2N1-x) in the Mn–N binary system. However, the crystal structure of the new bct phase differs from that of the ζ–phase, which has a hexagonal structure. Therefore, the bct phase is thought to differ from the ζ–phase. Further, the crystal structures of the new bct phase and the other Mn–N binary compounds, Mn4N, Mn3N2 and MnN, are similar; however, their lattice constants or N content values differ slightly. Through consideration of the magnetic properties, the new bct phase is assumed to be ferro- or ferrimagnetic. Note that Mn4N is ferrimagnetic, while the ζ–phase, Mn3N2, and MnN are antiferromagnetic at room temperature. The different lattice constants or the presence of Li may induce the magnetism of the bct phase.
The thermal stability of the bct phase was also investigated, and Fig. 8 shows the thermomagnetization curve of the 5-GPa sample. The sample magnetization increased slightly above 200℃ during the heating process, but almost disappeared at approximately 465℃. This value corresponds to the Curie temperature of Mn4N. Finally, Fig. 9 shows the XRD patterns of 5-GPa samples before and after heat treatment up to 300 and 550℃ under atmospheric pressure. The samples were heated at a rate of 10℃/min. The bct peaks remained present after heating to 300℃, but the a and c values in the bct–phase changed from 0.29186 to 0.29299 nm and from 0.39108 to 0.38697 nm, respectively. Judging from this result, the small magnetization increment at ~200℃ (Fig. 8) would be due to the changes in the bct–phase lattice parameters. After heating to 550℃, Mn3N2, MnO, and c–BN were primarily observed.

Thermomagnetization curve of Mn+LiNH2 treated at 1100℃ for 2 h under 5 GPa.

XRD patterns of Mn+LiNH2 treated at 1100℃ for 2 h under P = 5 GPa before heat treatment, and after heat treatment up to 300 and 500℃.
A new compound was synthesized from Mn and LiNH2 using a high pressure of 5 GPa, at a temperature of 1100℃ and for 2 h. The new phase had a bct structure (space group: I4/mmm, No. 139) with a = 0.29186 nm and c = 0.39108 nm. The 5-GPa sample exhibited coercivity and saturation magnetization of approximately 310.7 kA/m and 17.7 A∙m2/kg, respectively. The bct phase composition ratio was Mn:N = 2:1. It was found that Li plays a role in the formation of the bct phase, because this phase was not observed to develop from an Mn–N binary system. Precursor consisting LiNH2 for high-pressure synthesis was fount to be effective to explore new magnetic compound in Li systems.
This work was partly supported by the Japan Core Research for Evolutionary Science and Technology (CREST) project and Precursory Research for Embryonic Science and Technology (PRESTO) under the Japan Science and Technology Agency (JST).