2016 Volume 57 Issue 12 Pages 2072-2076
2016 Volume 57 Issue 12 Pages 2072-2076
ZnO-CaO-P2O5-Nb2O5 invert glasses, with ZnO substituted for CaO, were successfully prepared using a melt-quenching method. Their structures, dissolution behaviors, and antibacterial abilities were evaluated. Niobate switched its role of network former to modifier with increasing ZnO content, as Zn2+ ions preferentially coordinated with phosphate groups. In parallel, with increasing content of ZnO in the glasses, P-O-Zn bonds were formed, which crosslinked phosphate groups with ZnO acting as a network former. As a result, the glass-forming ability and chemical durability of the glasses were improved. The glasses showed antibacterial ability to gram-positive and -negative bacteria.
Biomaterial-centered infections are currently regarded as severe post-surgical complications and represent 5 to 10% of implant failures in dentistry1–3). These infections are usually characterized by a microbial colonization at the biomaterial surface, decreasing the likelihood of a successful integration of the biomaterial. With dental implants, it often leads to peri-implant diseases, such as peri-implant mucositis or peri-implantitis4). The latter is characterized by a destructive inflammatory response, affecting soft and hard tissues and contributing to a rapid loss of the supporting bone surrounding the implant4). The occurrence of these infections could potentially be reduced by applying an antibacterial coating on the abutment, part of the implant in contact with the gingival tissue4,5).
Upon controlled degradation of the coating, active chemical cues can be released and induce an antimicrobial effect. Metal ions, such as silver and zinc are known to exhibit such effect6,7). For instance, low dosage of Ag+ ion is clinically used to treat severe burn or as an active coating for catheter6,8). However, the use of Ag+ ion in dentistry is usually avoided as it reacts with sulfide residues present in saliva, darkening implants. As a consequence, zinc has drawn a considerable interest for dental implant as it exhibits similar antimicrobial activity than silver without any esthetic side effects9–11). In addition, it has been shown that Zn2+ ion is a good inhibitor of human dental plaque12). However, and similarly to silver, adverse effects on the healthy tissue can be generated from Zn2+ ion with a mild cytotoxicity at concentration above 10−4 mol/L13). Thus, it is important that the coating, which carries active species such as Zn2+ ion, has an excellent chemical durability with a controlled degradation behavior.
Such behavior can be obtained with niobium-containing phosphate invert glasses (Nb-PIGs)14,15). Nb-PIGs are composed of short phosphate group, e.g., ortho- and pyrophosphates (Qp0 and Qp1), cross-linked together with tetrahedral niobia NbO4 forming a glass network (P-O-Nb bonds)14,15). Compared to conventional phosphate invert glasses, the introduction of niobia significantly improved the chemical durability of the glasses14–16). Nb-PIGs can release ions such as Ca2+, P5+, Nb5+ and Na+ ions continuously in a controlled manner14). The ions-release can be finely tuned by varying the content of niobia. For instance, the total amount of ions released from the Nb-PIGs after 7 days of immersion decreased from 7.5% to 2.5% with an increase of Nb2O5 from 3 to 10 mol%14). In addition, Nb-PIGs showed promising biological response with stimulation of alkaline phosphatase activity (ALP) of osteoblast-like cells, a marker of differentiation17).
Radio frequency magnetron sputtering (RF-sputtering) could be used to coat dental implants with Nb-PIGs. Amorphous calcium phosphate (ACP) film was successfully deposited onto titanium using RF-sputtering18). The thickness of the ACP films were 0.5–1 μm with a tensile bonding strength between the film and titanium, above 60 MPa19,20). In addition, ACP film have smooth surface, which can diminish the colonization of the bacteria5). However, the bonding strength decreased to 30 MPa after ACP film on titanium was immersed in phosphate-buffered saline for 3 days, because of the low chemical durability of ACP film20). Thus, Nb-PIGs could potentially be excellent candidates for deposition of the amorphous films using a RF-sputtering method, due to their improved chemical durability compared to ACP. In addition, the incorporation of antibacterial inorganic ions can be achieved with Nb-PIGs and be released from the glass in a controlled manner as a function of the glass composition.
In this work, we investigated if ZnO could be incorporated in Nb-PIG and release in solution in a controlled manner upon immersion in aqueous media. We prepared ZnO-CaO-P2O5-Nb2O5 invert glasses by substituting ZnO for CaO, and evaluating the change in glass structure by Raman and nuclear magnetic resonance (NMR) spectroscopies. Their ion dissolution behaviors were monitored in Tris buffer solution, and their antibacterial properties were evaluated using Escherichia coli and Staphylococcus aureus.
Glasses with compositions of xZnO·(65 − x)CaO·30P2O5·5Nb2O5 (mol%, x = 0–65) were prepared using ZnO (99.5%), CaCO3 (99.5%), Nb2O5 (99.9%) and H3PO4 (85%, liquid) (all from Kishida Chemical Co.). The reagents were mixed with distilled water into a slurry, which was dried under an infrared lamp overnight and stored at 140℃. The mixture was melted in a platinum crucible at 1500℃ for 30 min and then quenched by pressing between two stainless-steel plates. The crystalline parts were removed manually. The resulting glass compositions were evaluated by energy-dispersive X-ray (EDX, JED-2300, JEOL Co.), in triplicate.
The glass transition temperature (Tg) and crystallization temperature (Tc, defined as the onset of crystallization) of the glasses were determined by differential thermal analysis (DTA, heating rate: 5 K/min, Thermoplus TG8120, Rigaku Co.). The glassification degree (GD) was calculated using (Tc − Tg)/Tg[K/K] as an indicator of the glass-forming ability21).
Laser Raman spectra were obtained to evaluate the glass structures in the 500–1300 cm−1 Raman shift region (NRS-3300, 532.02 nm, 6.4 mW, JASCO Co.). Solid-state 31P magic angle spinning nuclear magnetic resonance (MAS-NMR) measurement were also performed to evaluate the glass structures at a Larmor frequency of 242.954 MHz in a 3.2 mm rotor spinning at 15 kHz (JNM-ECA600II, JEOL Co.). Single pulse experiments with a 1.1 μs width, 5 s recycle delay and cumulated number of 256 scans were performed, using ammonium dihydrogen phosphate (NH4H2PO4, Kishida Chemical Co.) as a reference (1.0 ppm).
The glass frits were pulverized and sieved down to 125–250 μm in size. The chemical durability of the glasses was evaluated by immersing 15 mg of the glass powders 15 mL of 50 mM, pH 7.4, Tris buffer solution (TBS) at 37℃ for 1, 3, 5 and 7 days (n = 3). The concentrations of Ca2+, Zn2+, P5+, and Nb5+ ions at each time point were measured using inductivity coupled plasma atomic emission spectroscopy (ICP-AES, ICPS-7510, Shimadzu Co.).
The antibacterial ability of the glasses was examined using a shake method (Society of Industrial Technology for Antimicrobial Articles). Escherichia coli (E. coli, strain NBRC3972) and Staphylococcus aureus (S. aureus, strain NBRC12732) were used in this work.Nutrient broth (NB) medium was prepared by dissolving 18 g of nutrient broth ‘Eiken’ (E-MC35, Eiken Chemical Co.) in 1 L of distilled water, followed by autoclaving sterilization at 121℃ for 20 min. Glass powders were dry-sterilized at 180℃ for 90 min. Ten mg of glass powder was soaked in 10 mL 1/500 NB media with an initial concentration of 4 × 104 CFU/mL for E. coli and S. aureus. The media was subsequently incubated for 24 h at 37℃ in an orbital-shaking incubator (150 rpm). The numbers of colonies after cultivation were counted using a plate count agar method; agar plates were prepared from a standard agar (SA) medium. SA medium was prepared by dissolving yeast extract B2 (2.5 g, Oriental Yeast Co., Japan), Tryptone (5.0 g, Becton, Dickinson and Co.), D(+)-glucose (1.0 g, Wako Pure Chemical Co.) and agar (15 g, Wako Pure Chemical Co.) in 1 L of distilled water, followed by autoclaving sterilization at 121℃ for 20 min and poured into plastic petri dish.
The actual compositions are shown in Table 1 and were obtained by EDX. The relative concentration of ZnO, CaO, P2O5, and Nb2O5 varied by approximately 3, 6, 6, and 2 mol%, respectively, from their nominal values, regardless of the composition targeted. This disparity could be explained by the overlap in the signals given by phosphorus Kα (2.013 eV) and niobium Lα (2.166 eV).
| Glass code | ZnO | CaO | P2O5 | Nb2O5 | ZnO for CaO substitution (%) |
|---|---|---|---|---|---|
| 0Zn | - | 65 | 30 | 5 | 0 |
| - | (59.0 ± 0.9) | (36.4 ± 0.7) | (4.5 ± 1.4) | ||
| 8Zn | 5 | 60 | 30 | 5 | 7.69 |
| (5.6 ± 0.7) | (62.0 ± 2.2) | (29.3 ± 1.8) | (3.2 ± 0.6) | ||
| 23Zn | 15 | 50 | 30 | 5 | 23.08 |
| (14.8 ± 2.4) | (50.0 ± 1.2) | (32.5 ± 2.9) | (2.8 ± 0.6) | ||
| 31Zn | 20 | 45 | 30 | 5 | 30.77 |
| (19.3 ± 1.6) | (44.2 ± 0.5) | (33.1 ± 1.6) | (3.5 ± 0.3) | ||
| 46Zn | 30 | 35 | 30 | 5 | 46.15 |
| (33.5 ± 0.1) | (34.6 ± 0.5) | (33.5 ± 0.2) | (3.1 ± 0.3) | ||
| 62Zn | 40 | 25 | 30 | 5 | 61.54 |
| (35.9 ± 0.6) | (25 ± 0.3) | (35.0 ± 0.2) | (4.1 ± 0.2) | ||
| 77Zn | 50 | 15 | 30 | 5 | 76.92 |
| (47.8 ± 1.1) | (15.0 ± 0.3) | (33.8 ± 1.2) | (3.4 ± 0.2) | ||
| 100Zn | 65 | - | 30 | 5 | 100 |
| (61.4 ± 0.2) | - | (34.7 ± 1.2) | (4.0 ± 0.2) |
Figure 1 summarizes the Tg, Tc and glassification degree (GD) of the glasses extracted from the DTA curves. The Tg and Tc of the glasses decreased from 644℃ and 730℃ at 0% ZnO substitution, to 468℃ and 633℃ when CaO was fully substituted for ZnO, respectively. The GD increased from 0.09 to 0.22, at 0 and 100% ZnO substitution, respectively. This increase in GD could be related to an improvement of the glass forming ability since ZnO is an intermediate oxide with higher field strength than calcium, 0.49 and 0.33 (valence/Å2)23), respectively. The GD of 100Zn observed here was 0.22, which was comparable to this of zinc metaphosphate glass previously reported by Ouchetto et al. (0.25)21). It is likely that ZnO in Nb-PIGs acts as a network former, crosslinking short phosphate groups together24,25). To verify this assumption, the structures of the glasses were characterized by Raman spectroscopy and 31P MAS-NMR, as shown in Figs. 2 and 3, respectively.
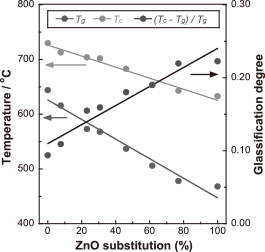
Glass transition temperature (Tg), crystallization temperature (Tc) and glassification degree (GD, (Tc - Tg)/Tg) for the glasses.

Laser Raman spectra for the glasses.
Typical Raman bands corresponding to Qp0 and Qp1 phosphate groups in the glasses were observed, including P-O-Psym stretching mode of the bridging oxygen in Qp1 (748–768 cm−1)24,26), (PO4)sym stretching mode of non-bridging oxygen in Qp0 (947–972 cm−1)24,26), (PO3)sym stretching vibration of the non-bridging oxygen in Qp1 (1040–1055 cm−1)24,26), and P-O stretching of the Qp1 chain terminator (1113–1190 cm−1)24). The peak top positions of phosphate groups were blue-shifted with increasing ZnO content, since the field strength of Zn is higher than that of the Ca23). Raman bands corresponding to niobia with tetrahedral (NbO4) and octahedral (NbO6) were observed with Nb-O vibrations of NbO6 octahedrons corner-linked in a three-dimensional network (645 cm−1)27,28), Nb-O vibrations of NbO4 tetrahedrons (855 cm−1)28), Nb-O vibrations in isolated NbO6 octahedral units (905 cm−1)27,28), and Nb = O stretching vibration mode of the NbO4 terminal bond (992 cm−1)29). With increase in ZnO content, the intensity of NbO4 and NbO6 bands decreased and increased, respectively. This decrease in coordination number from 6 to 4 suggests that niobia structurally changed its role from network former to network modifier28,30). In addition, the increase in glass forming ability, along with the higher affinity of zinc to coordinate with phosphate groups (higher electronegativity) confirmed that Zn2+ ion could crosslink phosphate groups together by forming P-O-Zn bonds and therefore acting as a network former24,25).
31P MAS-NMR spectra of the glasses are shown in Fig. 3(A). Two overlapped peaks between 10 and −25 ppm were detected and assigned to Qp0 and Qp1 units (structural representation given in Fig. 3(B))14,15). They are characteristic of phosphate invert glasses, which structurally consist of short phosphate chains and charge balanced with cations31,32). The experimental spectra were deconvoluted, assuming that Qp0 and Qp1 species followed a Gaussian distribution. The variation in integration of each deconvoluted species as a function of the ZnO substitution is given in Fig. 3(B). The peak-integrated portions associated with Qp0 and Qp1 groups decreased and increased linearly, respectively, with increasing ZnO content. This increase in connectivity could be explained by the different in coordination number of Zn2+ and Ca2+ ions, 4 and 8, respectively, in the glasses23). Thus, the substitution of CaO for ZnO and the associated decrease in coordination favored the polymerization of linear phosphate chains.

31P MAS-NMR spectra for the glasses (A), and peak integrated portions of phosphate groups of the glasses as a function of the ZnO substitution (B).
The chemical durability of the glasses to release Zn2+ ion was evaluated by immersion in Tris buffered solution (TBS). In this work, TBS was used since most of the physiological solutions, such as simulated body fluid (SBF)22) or cell culture media, are supersaturated with regards to calcium and phosphate ions, which may disturb the estimation of the ions-release from the glasses. Figure 4 shows the P5+, Nb5+, Ca2+, and Zn2+ ion-release profiles from the glasses into TBS after 1, 3, 5 and 7 days of immersion. With increase in ZnO content, the amount of Ca2+ and P5+ ions released from the glasses decreased from 0.40 and 0.38 mM at 0% ZnO substitution to 0 and 0.11 mM at 100% ZnO substitution, respectively. As expected, the concentration of Zn2+ ion gradually increased with increasing ZnO content, from 0.02 mM at 8% to 0.09 mM at 100% substitution, staying under 0.1 mM. The amount of Nb5+ ion released slightly decreased with increasing ZnO content. It has already been reported that the addition of ZnO and Nb2O5 in NaPO3-Al2O3 glass systems significantly improved their chemical durability when immersed in distilled water since they crosslinked phosphate groups together33). Similar effect was observed here resulting in increase in the chemical durability of the glasses as CaO was substituted for ZnO. In addition, the increase in the field strength of zinc as compared to calcium could also explain the improvement in chemical durability23).
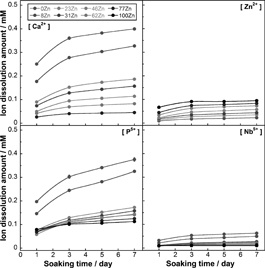
Ions release amount in TBS for Ca2+, Zn2+, P5+, and Nb5+ ions.
Finally, the antimicrobial activity of the glasses was tested against E. coli and S. aureus, using the using a shake method. This method was used as fundamental investigation tool to monitor the effect of the glass compositions. A more elaborate approach would be to use a film attachment method, which would closely resemble a RF-sputtered coating layer onto a dental implant. However, this is believed to be beyond the scope of this work. Figure 5 represents the number of colony forming unit (CFU) after being cultivated with the glass powders for 24 h. Bacterial growth was inhibited when the substitution for ZnO was above 23% for E. coli, and above 62% for S. aureus. ZnO-containing Nb-PIG showed controlled Zn2+ ion release, antibacterial ability and excellent chemical durability. This data shows that these glasses are excellent candidates as antibacterial coating on dental implants and could potentially decrease the occurrence of peri-implantitis infection.
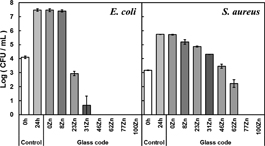
Number of E. coli (left) and S. aureus (right) after cultivation with the glass powders for 24 h. “0 h control” means the seeded number of E. coli and S. aureus, and the error bar means standard deviation.
The structures, dissolution behavior and antibacterial ability of ZnO-containing Nb-PIGs were investigated. The glass forming ability was improved by substituting ZnO for CaO. Zn2+ ion was preferentially coordinating with phosphate group in the glasses, crosslinking phosphate groups together and forming P-O-Zn bonds. As a result, niobia changed its role from network former to modifier. The chemical durability of the glasses was improved with increasing ZnO content, due to the formation of P-O-Zn bonds and the greater field strength of zinc compared to calcium. The glasses showed antibacterial ability to the gram-positive and -negative bacteria.
This work was supported in part by JSPS KAKENHI Grant Numbers 25249094, 26289238, and 267992. We are grateful Prof. Hashimoto and Prof. Takiguchi in Chiba Institute of Technology for helpful discussions of antibacterial experiments.