2017 Volume 58 Issue 1 Pages 23-25
2017 Volume 58 Issue 1 Pages 23-25
Sulfated anatase TiO2 nanocrystals were prepared by hydrothermal method. All samples were characterized by means of powder XRD and FT-IR. It is shown that all the as-prepared samples displayed the features of pure anatase phase. The surface hydration and sulfation of TiO2 became significantly enhanced as decreasing the particle size. The sulfated TiO2 nanocrystals showed photoactivities towards photodegradation of RhB under UV light irradiation, during which the sulfate species anchored on surfaces of anatase nanocrystals were not released into the reaction solutions.
The preparations of sulfated metallic oxides were generally carried out through the impregnation of TiO2 with H2SO4 or (NH4)2SO4, which needed the subsequent high temperature calcinations1). Therefore, there exist a determinant influence on the sintering kinetics, photochemical processes, and adsorption of gas phase components.2) Moreover, it is very difficult to control the quantity of sulfate groups using these impregnation methods, since surface sulfate species are generally thermodynamically unstable, and can be partially dissolved into water subsequently.3)
In this work, we present a solution method to prepare the highly crystalline TiO2 nanocrystals with defined surface chemistry. The chemical states of SO42− on TiO2 nanocrystals were specified. The results reported in this work are expected to extend to a broad class of oxide nanocrystals, which allows an in-depth understanding of the molecular-level interactions between oxide surfaces and surroundings4) for many applications including photocatalysis, environmental remediation, heterogeneous atmospheric chemistry, and aqueous geochemistry.5)
Sulfated TiO2 nanoparticles were directly prepared by a hydrothermal condition. Firstly, 4 g of TiOSO4·2H2O was dissolved in 140 mL distilled water under vigorously magnetic stirring. After stirring for about 10 h, a transparent solution was obtained, which was then transferred into Teflon-lined stainless steel autoclaves and reacted at a given temperature between 100 and 220℃ for 3 h. The precipitate was washed with distilled water for several times by vacuum filtration till no sulfate groups were detected in the residue solutions. All samples were named as T100, T140, T180 and T220, where the numbers denote the treatment temperatures at 100, 140, 180 and 220℃, respectivtly.
Phase structures of all samples were identified by powder X-ray diffraction at room temperature on a Rigaku Dmax 2500 PC apparatus (copper target, Kα, λ = 0.15418 nm). The particle size and shape of the suspension were observed using transmission electron microscopy (TEM) on a JEM-2010 apparatus with an acceleration voltage of 200 kV. FT-IR spectra of the samples were recorded using a KBr pellet technique on a Perkin Elmer Spectrum spectrograph.
In order to test the photocatalytic activity of the samples, photodegradation of rhodamine B (RhB) was carried out in the presence of nanocrystals at room temperature. Reaction suspension was prepared by adding 80 mg of sample into a 100 mL beaker containing 80 mL 10−5 M RhB. Prior to illumination, the solution was stirred for 7 h in dark to reach an absorption-desorption equilibrium of RhB. Subsequently, the suspension containing RhB and photocatalyst was irradiated under a 20 W low-pressure Hg lamp with a peak wavelength of 254 nm being used as a light source. 5 mL of the suspensions were sampled every 20 min. The UV–vis absorption spectra of the supernatant were measured using a Perkin Elmer UV WinLab Lambda 35 spectrophotometer. The molecular species in the suspension generated during the photodegradation process were analyzed with an ion trap mass spectrometry (ITMS) using DECAX-30000 LCQ Deca XP.
Figure 1(a) shows XRD patterns of the samples synthesized at different temperatures. All diffraction peaks can be indexed to an anatase phase. These results demonstrate that all as-prepared samples are composed of pure anatase phase. Average crystalline sizes were calculated using Scherrer formula from diffraction peaks (101). It is found that when the reaction temperature was increased from 100 to 220℃, the particle sizes of the as-prepared samples increase from 4.8 to 10.6 nm. The corresponding TEM image in Fig. 1(b) showed particle size of T100 is about 5 nm, which is similar to those calculated by XRD peak broadening, indicating a good dispersity.
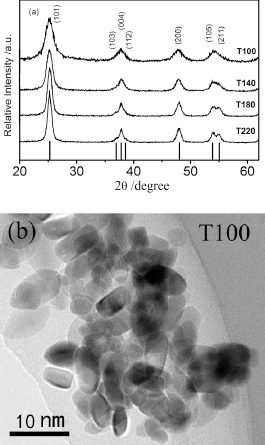
(a) XRD patterns of the samples prepared at given temperatures. Vertical bars below the patterns represent the standard diffraction data from JCPDS file for anatase (No. 21-1272). (b) TEM image of sample T100.
Surface chemical states of the samples were investigated by FT-IR spectroscopy. All the as-prepared TiO2 nanocrystals gave a set of characteristic absorptions of SO42− in the range of 1250–970 cm−1, namely, two obvious absorption bands (ν2 and ν3) and two shoulder peaks (ν1 and ν4) in Fig. 2. For 4.8 nm TiO2, the absorptions of SO42− were located at about 1206 (ν1), 1127 (ν2), 1047 (ν3), and 990 cm−1 (ν4), respectively, which are associated with the chelating bidentate SO42−.6) When the particle size increased to 10.6 nm, both absorptions ν1 and ν4 showed systematic red shifts to 1178 and 971 cm−1, respectively, while those of ν2 and ν3 did not have apparent shifts but remained at 1122 and 1055 cm−1. This observation demonstrated the presence of unidentate SO42− for larger TiO2 nanocrystals.6) These chemical states of sulfate species are apparently different from those of bridged bidentate SO42− species prepared by traditional impregnation method.7) The characteristic band for asymmetric stretching of the S-O bond is absent in the range from 1200 and 1235 cm−1, which however had already been observed for the sulfated TiO2 prepared by traditional methods.7) The position shift is associated with the transition of bidentate coordination to unidentate one. Therefore, all the as-prepared TiO2 nanocrystals can be roughly modeled as a TiO2-like core being sufaces bonded by bridging hydroxyl and sulfate groups.
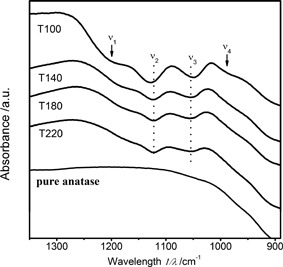
FT-IR spectra of as-prepared TiO2 nanocrystals and pure anatase TiO2.
Photocatalytic activities of the as-prepared samples were monitored via degradation of RhB in water solution under UV irradiation (254 nm). Figure 3 illustrated the absorption spectral changes of RhB solution in the presence of as-prepared sample dispersions. As indicated in Fig. 3, RhB solution showed a characteristic primary absorption at about 554 nm, and the positions of the primary absorption systematically shifted towards lower wavelengths with prolonging the periods of irradiation.
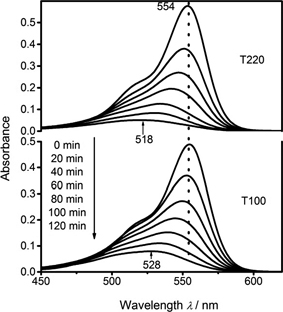
UV-Vis absorption spectra of RhB aqueous solution with as-prepared samples (T220 and T100).
Typical ITMS spectra of RhB solution in the presence of 6.7 nm as-prepared sample after UV irradiation were illustrated in Fig. 4. It is seen that the solution consisted of six types of components. One of the components is the initial RhB dye at m/z = 443.5. The other five components were identified as DMRh, DRh, MMRh, MRh, and rhodamine (Rh). It is interesting to note that no peak was observed at m/z = 48 in Fig. 4, which indicates that none of sulfate groups was released in the solution from the sample surfaces during the UV-irradiation. This result is fundamentally important since the present methodology gives stable surface sulfation, while surface sulfation achieved by traditional sol-gel methods is unstable and dissolved into water8). Therefore, the methodology represents an effective route to stabilize the sulfate species on TiO2 nanocrystals for highly chemical activities, which might be useful to the understanding of activities of sulfated TiO2 and many other oxides.
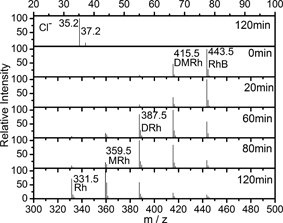
Relevant mass spectra of the intermediates during degradation of RhB molecules with as-prepared samples (T180) under UV light irradiation.
Sulfate groups might be bonded in a special configuration to the hydrated surfaces of anatase nanostructures to achieve highly stable surface sulfation. There are two possible coordinations for sulfate groups to be bonded with the surfaces of anatase nanocrystals: outer- and inner-layer sulfate complexes. As illustrated in Figs. 5(a) and (b), outer-layer sulfate groups are directly bonded to surface water, therefore it is thought that the chemical nature of these sulfate groups would be similar to aqueous sulfate with Td symmetry to show two characteristic IR vibrations, ν1 and ν39). While for the as-prepared samples, more than two IR bands were observed (Fig. 2), which indicated that the sulfate groups should not be in merely outer-layer form. Alternatively, when SO42− is present in inner-layer form (Figs. 5(c) and (d)), the symmetry of S-O4 tetrahedron would be lowered and ν3 would be spiltted. For inner-layer sulfate complexes, unidentate forms (Fig. 5(c)) can be more stable than bidentate ones (Fig. 5(d)). This is because sulfate groups in bidentate form are coordinated with two Ti ions and thus might show a larger O-S-O bond angle in comparison with the O-Ti-O bond angle of anatase surfaces. As a consequence, sulfate tetrahedron would become severely distorted, leading to a significant increase in total energy of the system. Any external driving force could lead to the transformation of bidentate coordination to unidentate one. For the present methodology, high reaction temperature may lead to breakage of certain S-O bonds in bidentate coordination to transform into unidenate coordination to decrease the total system energy. This is why IR peaks ν1 and ν4 shift towards lower frequency sides as increase of particle size. It should be mentioned that, with increasing the reaction temperature, the uni-/bidentately octahedral in inner-layer form would be the majority of the surface species, and their desorption can become thermodynamically unfavorable,10) in consistent with our IR spectrum (Fig. 2). Comparatively, when using traditional impregnation methods or sol-gel methods, stabilization of surface sulfation is difficult to achieve, since the sulfate species absorbed on the surfaces of TiO2 nanocrystals are primarily in the form of out-layer coordinations.
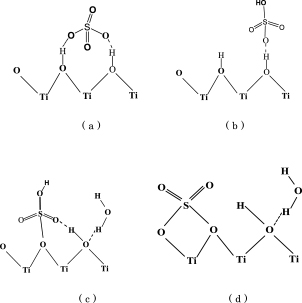
Coordination model of sulfate groups bound to the surfaces of anatase nanocrystals.
This work reports a solution chemistry to stable and highly photoactive surface sulfation of TiO2 nanocrystals. Both types of chelating bidentate- and unidentate sulfate groups were found to coexist on the surfaces of the samples. All as-prepared TiO2 nanocrystals showed photoactivities towards photodegradation of RhB under UV light irradiation, and none of sulfate groups was released in the solution from the sample surfaces during the UV-irradiation.
This work was supported by a grant from Weifang University of Science and Technology. We express our gratitude to professor Xiaoqing-Qiu for careful reading of the manuscript and technical assistance.