2017 Volume 58 Issue 10 Pages 1458-1462
2017 Volume 58 Issue 10 Pages 1458-1462
We investigated the relationships between the centrifugal force and the disc size and rotation speed during the production of fine particles. In this study, an empirical formula was developed with the goal of obtaining an average particle size of D50. Moreover, particle production was performed at a disc rotation speed of at least 100,000 rpm, which was greater than that reported in previous studies. Our results showed that with Sn-13 mass%Sb (called Sn-13Sb), the average particle size produced at a disc rotation speed of 115,000 rpm was 12.8 μm, with 96.5% of the particles smaller than 25 μm.
Solder materials are used in the assembly of electronic circuit boards. In the 1990s, stronger regulations regarding the use of lead were established and today, 80% of the solder used worldwide is composed of materials that do not contain lead.1) For the reflow process, the particle size of the solder pastes used in attaching chip components and ball grid arrays (BGAs) is primarily 25–45 μm. However, electronic components are constantly being developed with ever-denser circuit boards. This is a result of the miniaturization and weight reduction of electric devices. Although the chip size mainly used in applied fields is 0603 (0.6 × 0.3 mm), this will change to 0402 and 0201. Therefore, a demand for a particle size of no more than 25 μm or even no greater than 12 μm is expected. BGA components are also expected to be reduced in thickness from 0.3 to 0.2 mm.
Centrifugal atomization is a common method for manufacturing solder particles. It enables particles to be produced that have lower oxidation and higher sphericity than those produced by the current gas atomization method.2) In the centrifugal atomization method, molten metal is dropped onto a disc that spins at a high speed, where it is atomized by the centrifugal force. Studies on the manufacture of solder particles through centrifugal atomization have advanced based on theories regarding the atomization of droplets using a centrifugal force. These studies have shown that higher disc speeds are needed to produce smaller particles.3–5) Moreover, experiments have been conducted using disc rotation speeds of R = 15,000–40,000 rpm, with the aim of efficiently manufacturing particle sizes of approximately 50 μm.6) Further, fine Sn-3Ag-0.5Cu powder particles of 45 μm or smaller containing oxygen at less than 100 ppm have been synthesized and could be applied to lead-free solder.7) Furthermore, a hybrid method using gas atomization has been studied in order to manufacture particles with diameters no greater than 10 μm. Particles with an average diameter of 10.8 μm have been identified at disc rotation speeds of 30,000 rpm. In addition, it has been observed that 45.5% of the particles had a diameter no greater than 25 μm.8)
To obtain a narrow range of particle sizes, it is necessary to achieve stable atomization by maintaining a uniform thickness for the liquid coating on the surface of the disc. Accordingly, studies have suggested that a narrower range of particle sizes can be achieved by not using gas atomization, which destabilizes the coating thickness on the surface of a disc. For this reason, in our study, we examined the relationships between the centrifugal force and the disc size and rotation speed in the manufacture of finer particles. Moreover, studies have confirmed that the theoretical and experimental values are consistent for particles manufactured using a disc rotation speed of at least 100,000 rpm. However, this information has not been reported in previous studies.
A schematic of the centrifugal atomization equipment used in our study is shown in Fig. 1. We melted the solder alloy by heating it to a temperature of 30℃ above its melting point in a heating furnace in a nitrogen atmosphere. The furnace was installed above an atomization chamber. The molten solder was dropped onto the center of a high-speed spinning disc from a fluid nozzle installed on the underside of the heating chamber and was atomized by the centrifugal force on the disc. The atomized droplets formed into spherical particles as a result of the surface tension in the atomization chamber. The solidified particles were then collected in a vessel installed at the base of the chamber. The oxygen partial pressure in the atomization chamber was maintained at 10–30 ppm.

Apparatus of centrifugal atomization.
The tests were conducted in two stages. First, the disc diameter necessary to affect the particle size was determined using a standard motor with a maximum rotation speed of 60,000 rpm. Micronization was investigated using disc diameters of 20, 30, 35, and 40 mm. Next, a motor with a maximum rotation speed of 120,000 rpm was substituted, and fine particulate matter was manufactured with this disc spinning at a high speed.
Although solder materials have been developed with various kinds of alloys, the following solders, which are expected to have increased use in the manufacturing industry, were employed in our study: Sn-3.5 mass%Ag-0.5 mass%Bi-5.9 mass%In-0.8 mass%Cu (hereinafter called Sn-3.5Ag-0.5Bi-5.9In-0.8Cu), which has a highly reliable joining capacity,9) and Sn-13Sb, which has a high heat resistance. We used a laser diffraction-scattering type particle size distribution measuring device (MicrotracBEL Corp., model MT3300EX II) to measure the diameter of the manufactured particles. D50 was calculated as the average particle size, and D75/D25, which is the ratio of D75 to D25 particles, was calculated as the particle size distribution width. Particle shape observations were conducted using a scanning electron microscope (Hitachi High-Technologies Corporation, model SU-70). The oxygen content of the particles was measured with an inert gas infusion/non-dispersive infrared absorption method using nitrogen and oxygen analysis equipment (HORIBA, Ltd., model EMGA-920).
Sn-3.5Ag-0.5Bi-5.9In-0.8Cu was heated to 230℃ and slowly dropped onto the disc at 0.88 kg/min. Fig. 2 shows the variations in the D50 average size of the atomized particles based on the disc diameter and rotation speed. The D50 average particle size decreased with an increase in the disc rotation speed or disc diameter. The average size of the particles atomized through centrifugal atomization is expressed in the following equation.10)
| \[ {\rm D}50 = (6.0 \times 10^{5}/2 \pi {\rm R}) \times \surd (12\gamma/\rho{\rm D}) \times 10^{2} \] | (1) |
| \[ {\rm D}50 = 2.70 \times 10^{6}/({\rm R} \surd {\rm D} ) \] | (2) |
| \[ {\rm D}50 = 1.07 \times 10^{6}/{\rm R}^{0.975} \] | (3) |
| \[ {\rm D}50 = 1.35 \times 10^{6}/{\rm R} \] | (4) |

Effect of disc rotation speed on median particle size.
| 20mm | 30mm | 35mm | 40mm | |
|---|---|---|---|---|
| 40000 rpm | 6.5 | 10.8 | 1.8 | |
| 45000 rpm | 3.9 | 4.9 | 0.1 | |
| 50000 rpm | 22.2 | 7.6 | 7.1 | 1.7 |
| 55000 rpm | ||||
| 60000 rpm | 14.3 | 1.8 |
However, when the disc diameter was 20 mm, the particles obtained were 14% larger than the calculated particle size. The centrifugal force F(N) applied to the droplets of molten solder on the disc and the surface tension S(N) on the disc that restrained the droplets are shown, respectively, in the following two equations.
| \[ {\rm F} = (\pi /6)\rho {\rm d}^{3} {\rm r}\omega^{2} \] | (5) |
| \[ {\rm S} = \pi{\rm d}\gamma \] | (6) |
Figure 3 shows the particle size distribution width D75/D25. This affects the yield rate, which is important when manufacturing solder for industrial use. The particle size distribution width D75/D25 increases with the disc rotation speed. However, this width is different with a disc diameter of 20 mm compared to that with a 30, 35, or 40 mm disc. The values with these last three disc diameters are in the range of 1.42–1.6, whereas that for the 20 mm disc is larger at 1.95–2.11. To clarify the difference between these two classes of disc diameters, the particle size distributions for disc diameters of 20, 30, and 40 mm at a disc rotation speed of 50,000 rpm are shown in Fig. 4. When the disc diameter is 30 or 40 mm, a higher peak for the particle size distribution occurs, whereas the peak of the particle size distribution for a disc diameter of 20 mm is lower. Moreover, although the maximum particle size distribution is approximately 100 μm if the disc diameter is 40 mm, the distribution is wider for disc diameters of both 20 and 30 mm, with a maximum size of 200 μm. These particle size distributions reveal that if the disc diameter is longer, a narrow particle size distribution occurs. Because the maximum value of the particle size distribution is larger, if the disc diameter is smaller but the disc rotation speed is unchanged, the particle size may need to be larger for the centrifugal force F to match the surface tension S.

Effect of disc rotation speed on particle size distribution.

Particle size distributions of powders under different disc diameter.
These results show that larger discs are more appropriate to manufacture fine particle solder. However, Kusaka reported that if the disc is larger, the particle size distribution width D75/D25 will be wider as a result of the separation of droplets from the inner circumference of the disc, as well as the circumference of the disc itself.4) The particle size distribution widths obtained in this study for disc diameters in the range of 20–40 mm are shown in Fig. 3, and we can assume that for discs larger than 40 mm, the particle size distribution width D75/D25 will be even greater.
3.2 Relationship between centrifugal force and particle sizeDroplet diameter d is expressed as the balance between centrifugal force F and surface tension S. If centrifugal force F exceeds surface tension S, the material separates from the disc and forms droplets. Therefore, from (5) and (6), droplet diameter d is derived as follows:
| \[ {\rm d} = \surd 6 \gamma/\rho{\rm r}\omega^{2} \] | (7) |
| \[ {\rm D}50 = 5.92 \times 10^{6}/({\rm R}\omega^{2})^{-0606} \] | (8) |

Effect of Rω2 on median particle size in case of Sn-3.5Ag-0.5Bi-5.9In-0.8Cu.
To describe the trend for a solder with a different alloy composition, experiments were conducted using Sn-13 mass%Sb, which has a surface tension γ/density ρ value that is close to that for Sn-3.5Ag-0.5Bi-5.9In-0.8Cu, with a disc diameter of 40 mm and rotation speeds between 10,500 and 45,000 rpm. The surface tension γ/density ρ value of Sn-3.5Ag-0.5Bi-5.9In-0.8Cu is 6.68 × 10−5 compared to 6.74 × 10−5 for Sn-13Sb, with the latter having a surface tension γ of 0.49 (N/m) and a density ρ of 7.27 × 103(kg/m3). Figure 6 shows the particle diameter D50 plotted against Rω2. These have a linear relationship that can be expressed as follows:
| \[ {\rm D}50 = 6.97 \times 10^{5}/({\rm R}\omega^{2})^{-0.502} \] | (9) |
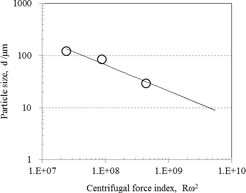
Effect of Rω2 on median particle size in case of Sn-13Sb.
Therefore, to achieve a D50 average particle size of 12 μm with both Sn-3.5Ag-0.5Bi-5.9In-0.8Cu and Sn-13Sb, a disc rotation speed of approximately 110,000 rpm is advisable.
3.3 Particle manufacture using high-speed disc rotationFigure 7 shows the D50 average diameter of the Sn-13Sb particles atomized at a disc rotation speed of 115,000 rpm with a disc diameter of 40 mm, and Fig. 8 shows the particle size distribution width D75/D25. Here, the experimental data from section 3.2 were used for disc rotation speeds of 45,000 rpm and less. In our study, the D50 average particle size for a disc rotation speed of 115,000 rpm was 12.8 μm, which was somewhat larger than expected and 8.5% greater than the D50 average particle size value of 11.8 μm calculated using (7). The reason for this may be slippage between the rotating disc and molten solder. Because slippage can be quite high when the disc rotates at a high speed, the ω value in (5) would be lower. For that reason, for centrifugal force F to match surface tension S, it is necessary to balance this with a large radius r or droplet diameter d. However, the maximum radius from the center of the disc is 40 mm. Therefore, this shortfall must be compensated by droplet diameter d. From this point forward, to produce even finer particles, taking countermeasures against slippage effects will be necessary because the slippage effect increases with the disc speed. In addition, the particle size distribution width D75/D25 increases with the disc rotation speed. However, we found a good value of 1.59 at 115,000 rpm in our study. Therefore, we could avoid the larger particle size distribution widths observed with the 20 mm diameter disc.

Effect of disc rotation speed on median particle size.

Effect of disc rotation speed on particle size distribution.
Table 2 lists the yields for particles with diameters of 12 μm or less and 25 μm or less. Although the yield for particles with diameters of 12 μm or less was 1.5% at 45,000 rpm, this increased to 42.6% at 115,000 rpm. Moreover, the yield for particles with diameters of 25 μm or less was as high as 96.5% at 115,000 rpm. A conventional hybrid method using gas atomization achieved a yield of 45.5% for particles with diameters of 25 μm or less at 30,000 rpm. When using the high-speed centrifugal atomization method, the yield for particles that were 25 μm or less was doubled compared with that using the conventional method. Further, particles of 12 μm or less could also be obtained in high yield. By increasing the disc rotation speed without using gas atomization, it was possible to stabilize the molten solder thickness on the disc surface, which resulted in a narrow particle size distribution.
| Disc rotation speed (rpm) | 10500 | 20000 | 45000 | 115000 |
|---|---|---|---|---|
| Yield of-12 μm (%) | 0 | 0 | 1.5 | 42.6 |
| Yield of-25 μm (%) | 0 | 0.2 | 29.9 | 96.5 |
Figure 9 shows scanning electron microscopy (SEM) photographs of solder particles manufactured with disc rotation speeds of 20,000 and 115,000 rpm. As these SEM photographs reveal, the particle diameters at 20,000 rpm were approximately 100 μm. However, at 115,000 rpm, many spherical particles were manufactured that were no greater than 25 μm in diameter.

Particle size when disc rotation speed is changed.
The oxidation state of solder particles used in industry will affect their efficacy. Therefore, the oxygen content of the manufactured solder particles was measured and plotted in Fig. 10. In our study, the oxygen content increased with a decrease in the D50 average particle diameter and increase in the specific surface area of the particles. The oxygen partial pressure was maintained at a level of less than 30 ppm in the atomization chamber so that hardly any oxygen was present. However, because the solder contacted oxygen during processing after being removed from the atomization chamber, oxygenation may have occurred. For the industrial use of solder particles smaller than 25 μm, consideration should be given to their handling after atomization.

Relationship between particle size and oxygen content.
To further refine the particles, it is necessary to use even faster disc rotation speeds or further reduce the surface tension γ/density ρ in (7). However, in order to increase the disc rotation speed, it is necessary to solve the problem of slippage between the molten solder and rotating disc. Moreover, because the surface tension and density are inherent characteristics of metals, we do not believe that these can be dramatically improved. Therefore, the production of finer particles by means of centrifugal atomization may be limited to an average particle size D50 of approximately 10 μm.
This study examined particles produced by rotation with an Rω2 value on the order of 109 using an electric motor capable of a rotation speed greater than 100,000 rpm. This revealed the following:
a. For Sn-3.5Ag-0.5Bi-5.9In-0.8Cu, D50 = 5.92 × 106/(Rω2)-0.606
b. For Sn-13Sb, D50 = 6.97 × 105/(Rω2)−0.502
The disc rotation speeds needed to manufacture particles with D50 diameters of 12 μm and disc diameters of 40 mm were similar for two types of solder materials: 106,400 rpm for Sn-3.5Ag-0.5Bi-5.9In-0.8Cu and 119,000 rpm for Sn-13Sb.