2017 Volume 58 Issue 12 Pages 1702-1707
2017 Volume 58 Issue 12 Pages 1702-1707
The recycling process of the coarse machined chips from the commercial Ti-6%Al-4%V (Ti-64) alloy products to fine powders available for powder metallurgy (PM) components was established. The brittle TiH2 compounds formation in Ti chips via heat treatment in hydrogen gas atmosphere significantly improved their milling ability. TG-TDA and XRD analysis suggested the hydration and dehydration behavior of pure Ti and TiH2 powders. The suitable hydration temperature over 873 K in H2-Ar mixed gas successfully caused TiH2 compounds synthesis in Ti-64 chips, and resulted in reproduction of fine Ti-64 powders with a median particle size of 120 µm from the machined spiral chips by mechanical milling process. The green compact of reproduced Ti-64 powder after vacuum sintering at 1273 K showed a relative density of about 93%, larger than that of the sintered material in using the commercial Ti-64 powder. The hydrogen and oxygen contents of the sintered specimen using reproduced Ti-64 powder were satisfied with JIS-60. Accordingly, the machined Ti alloy chips were directly recycled as valuable starting materials to reproduce PM components via combination of hydration-milling-dehydration process.
This Paper was Originally Published in Japanese in J. Jpn. Soc. Powder Powder Metallurgy 64 (2017) 81–87.
Since titanium (Ti) has some advantages such as high specific strength and Young's modulus, excellent corrosion resistance and biocompatibility, they are widely used in the various industries; for example, chemical plants components, heat exchangers and power station condensers. Recently the use of Ti materials in the airplane components, biomaterials products and medical devices remarkably increases. From a materials cost reduction point of view, the effective recycling and reproduction process of wasted Ti materials should be established because Ti is one of expensive rare metals. In the Ti materials industries, their scrap materials are generally reused to fabricate Ti ingots via re-melting process; for example, Ti scraps react with iron chloride wastes to form titanium tetrachloride (TiCl4) used as starting materials in the Ti smelting process1). It is, however, known that the recycling process of machined Ti chips has not been completed yet when they are produced in machining their components and products. The machined chips of the active light metals such as aluminum (Al) and magnesium (Mg) are never served to the re-melting process, but consolidated in solid-state by the mechanical pressing technique using plastic working process to form their compact billets2–6). In this solid-state recycling process of the above machined chips, the severe plastic forming process is also effective to the grains refinement and uniform dispersion of intermetallic compounds, and results in the significant improvement of the mechanical properties of the recycled materials, compared to the original virgin materials2,4). When this solid-state recycling process is applied to the active metal chips, a smaller specific surface area of the chips is more effective to inhibit the oxidation at the materials surface. That is, the fragmentation and refinement of the chips during the recycling process is not suitable. On the other hand, since the size and morphology of the machined chips are various, in general, a filling rate of the chips in the compaction die will be unstable. As a result, the compaction conditions should be optimized by case in use of the different chips. In particular, the apparent density of the machined chips is 30~50% smaller than that of the commercial metal powders. Therefore, it is difficult to fabricate long size billets from these chips via die compaction process. Ti machined chips also easily react with oxygen atoms. It is, however, well known that the synthesis and decomposition of titanium hydrides occur by heat treatment of Ti chips in the relatively low temperature7). In addition, titanium hydrides are brittle, thermally stable at room temperature, and hardly cause oxidation. It means that the formation of brittle hydrides in Ti chips enhances a fragmentation and refinement of the chip by mechanically milling process without oxidation reaction. That is, safe to operate the milling process of the titanium hydrides in air. According to the above characteristics, the Ti powders are commercially produced from sponge Ti materials by hydride-dehydride (HDH) process8,9).
The objective in this study is to establish the solid-state recycling process of Ti wastes to effectively reproduce Ti powder availably used as raw starting materials for PM components. When the hydration heat treatment is applied to the machined chips of the commercial Ti-6%Al-4%V (in mass%), named as Ti-64, the effect of hydrides formation on the milling ability is investigated. Furthermore, the mechanically milling process and HDH heat treatment conditions are optimized to produce Ti-64 raw powders with limited impurities of hydrogen, oxygen, nitrogen and carbon elements. Finally the compactibility and sinterability of the reproduced Ti-64 powder from their chips are also evaluated by comparison with the commercial powder.
The thermal decomposition behavior of the commercial titanium hydrides (TiH2) powders (TCH450: Toho Technical Service Co.) was investigated by using Thermogravimetry-Differential Thermal Analysis (TG-DTA) equipment (DTG-60, SHIMADZU) to understand the hydration and dehydration reaction of Ti materials. The analysis results are valuable references to determine the hydration temperature of Ti-64 machined chips. A standard specimen; Al2O3, heating rate; 20 K/min., maximum temperature; 1273 K, and flowing rate of argon gas (purity; 99.999%); 150 ml/min. are used as experimental conditions in TG-DTA. Thermal reaction behavior of pure Ti powder (TC-450: Toho Technical Service Co.) was also evaluated under the same analysis conditions. A median particle size of TiH2 and pure Ti powders measured by laser particle size analyzer (LA-950, HORIBA) are 18.8 μm and 21.9 μm, respectively. The chemical compositions of each powder are shown in Table 1. TiH2 powder contains 3.5~4 mass% hydrogen, and the other impurities oxygen are satisfied with their contents of pure Ti grade-4 (CP-Ti) specified in Japanese Industrial Standards (JIS). The hydrogen content of pure Ti powder is 0.04 mass%, and larger than that of pure Ti grade-4 because it is commercially produced by HDH process.
| Chemical compositions [mass%] | ||||||
|---|---|---|---|---|---|---|
| H | O | N | Fe | C | Ti | |
| TiH2 raw powder (TCH450) | (3.5~4) | (0.13) | 0.02 | 0.03 | Bal. | |
| Pure Ti powder (TC-450) | 0.04 | 0.20 | 0.02 | 0.03 | <0.01 | Bal. |
| JIS grade 4 (CP-Ti) | <0.013 | <0.40 | <0.05 | <0.50 | <0.08 | Bal. |
Figure 1 indicates Ti-64 machined chips under the different conditions (type A and B). After putting both chips (weight ratio, A:B = 50:50 mass%) into the vessel, they were heat treated in hydrogen (purity; 99.999%) and argon mixed gas atmosphere by using a tube furnace (ARF-2-500, Asahi). The flowing rate of H2 and Ar gas in the furnace were 2 L/mi. and 1 L/min., respectively. The temperature inside the furnace was controlled at 673 K~1073 K, and heat treatment time was 30 min. After heat treatment, the chips were cooled to room temperature in the furnace by flowing Ar gas to inhibit oxidation of specimens. X-ray diffraction (XRD) analysis was applied to the heat treated chips to identify the hydrides formation by using X-ray diffractometer (XRD-6100, SHIMADZU).

Two kinds of Ti-64 machined chips used as starting raw materials in this study.
Ten grams specimens of each Ti-64 chip after hydration treatment were filled into ZrO2 jar installed in the planetary ball milling equipment, and crushed and grinded by 120 g ZrO2 media balls with 10 mm diameter during milling process. The rotating speed of the jar was 200 rpm, and the milling time was 10~60 min. The oxygen, nitrogen and hydrogen contents of the milled chips were measured by using oxygen, nitrogen and hydrogen elemental analyzer (EMGA-830, HORIBA). The average value of three measurements was used as an analysis result of each element in this study. The dependence of a median particle diameter (D50) measured by laser particle size analyzer on the hydration temperature and milling time was investigated. Regarding the sinterability of each milled Ti-64 chip, each green compact was sintered at 1273 K in vacuum furnace, and changes in the relative density changes and O/H contents of the sintered bodies were measured. At the same time, the commercial Ti-64 alloy powder used as a reference material was also compacted and sintered under the same condition.
In general, the hydrides synthesis reaction of Ti and Mg is reversible, and their reaction behavior analysis is available to establish the materials design of hydrogen absorbing alloys10,11). First of all, to understand the hydration and dehydration reaction of Ti-64 chips, the thermal decomposition behavior of the commercial TiH2 powder was investigated by using TG-DTA equipment. As shown in Fig. 2, TiH2 powder obviously indicates two large exothermic peaks at 773 K and 873 K, and a small exothermic at 1173 K was detected. The latter peak was also observed in case of pure Ti powder. When considering this exothermic temperature of 1173 K was close to β-transus (1158 K)12), this small exothermic peak is due to α→β phase transformation during heating. On the other hand, regarding the former two large exothermic reactions, a TGA profile of TiH2 shows a large weight reduction at the same temperature, and the TGA of about 3 mass% was almost equal to the hydrogen content of TiH2 powder (3.5~4 mass%). These results suggest the large exothermic peaks were due to a dehydration reaction of TiH2 phases. Furthermore, according to the thermodynamic data13), the standard free energy of TiH2 at 1047 K is 0. That is, TiH2 powder thermally decomposed in 1047 K or more, and Ti and H2 gas exist individually. In the ΔDTA profile shown in Fig. 2, the above exothermic reaction completed at about 1073 K, and this was close to the thermal decomposition temperature of TiH2 (1047 K) as mentioned above. Therefore, it is concluded these large exothermic peaks were caused by a dehydration of TiH2 powder. The previous studies14,15) reported pure Ti materials (α-Ti) production from TiH2 as shown in the below reaction;
| \[ {\rm TiH}_{2} \to {\rm TiH}_{X} (\delta) + {\rm H}_{2} {\uparrow} (1.5 < X < 2) \] | (1) |
| \[ \delta \to \beta_{\rm H(High)} + {\rm H}_{2} {\uparrow} \] | (2) |
| \[ \beta_{\rm H(High)} \to \beta_{\rm H(Low)} + {\rm H}_{2} {\uparrow} \] | (3) |
| \[ \beta_{\rm H(Low)} \to \alpha_{\rm H} + {\rm H}_{2} {\uparrow} \] | (4) |

Thermogravimetry-differential thermal analysis profiles for TiH2 and pure Ti powders as a function of temperature, measured in Ar gas atmosphere under heating rate of 20 K/min.
Ti-64 chips were heat treated at 673 K, 873 K and 1073 K for 30 min. in the tube furnace with flowing H2-Ar mixed gas, and their hydrogen content measurement and hydrides identification by XRD were carried out. Figure 3 shows the hydrogen content of as-received chips and those after hydration treatment at 673 K was 66 ppm and 63 ppm, respectively. In addition, both samples revealed no significant difference of TGA. It is obvious that no hydration reaction occurred in the Ti chips when the heat treatment at 673 K in H2 gas atmosphere was employed. On the other hand, when heat treatment temperature at 873 K or 1073 K was employed, the hydrogen content amounted to 0.65~0.68 mass%, which was about 100 times as much as that of as-received Ti-64 raw chips. In addition, the difference of hydrogen content of both heated chips was very limited. According to the TG-DTA profile shown in Fig. 2, a significant increment of hydrogen content was due to the reaction between Ti-64 chips and H2 gas during heat treatment over 873 K. XRD profiles shown in Fig. 4 also indicate that Ti-64 chips after heat treatment at 873 K and 1073 K obviously showed TiH2 diffraction peaks while no TiH2, but only α-Ti peak was detected in the samples treated at 673 K as well as raw chips. Accordingly, the hydration heat treatment over 873 K is effective to synthesize brittle hydrides in Ti-64 chips.
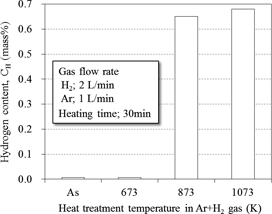
Hydrogen content of Ti-64 machined chips after heat treatment in H2-Ar mixed gas atmosphere at 673 K, 873 K and 1073 K, compared with raw chips.

XRD profiles of Ti-64 machined chips after heat treatment in H2-Ar mixed gas atmosphere at 673 K, 873 K and 1073 K, compared with raw chips and TiH2 powder.
To evaluate the effect of in-situ formed brittle hydride compounds on the milling ability of Ti-64 chips after hydration treatment, the relationship between the milling time and mean particle size of the above grinded chips with different hydrogen content was investigated. The milling times of 10 min., 30 min. and 60 min. were used in the planetary ball milling test. Figure 5 shows morphology changes of each chip after milling for 10~60 min. In case of Ti-64 raw chips (a) and with 673 K hydration treatment (b), both samples after 10 min milling process showed spiral shape as same as the chips before milling. After 30 min and 60 min milling process, the coarse grinded powder was visually observed. On the other hand, Ti-64 chips with hydration process at 873 K (c) and 1073 K (d) even after 10 min. milling revealed no original spiral shape and some of coarse particles, but most of them were fine. Longer milling treatment in 60 min. produced much fine Ti-64 powder compared to the samples with lower hydrogen contents shown in (a) and (b). The collect rate, R (%) expressed in the below equation was employed to quantitatively evaluate the milling ability of Ti-64 chips with different hydration temperature;
| \[ R = (W_{0} - W_{s})/W_{0} \times 100(\%) \] | (5) |

Morphology changes of heat treated Ti-64 chips after ball milling at 10 min, 30 min and 60 min. (a) as-received raw chips, (b) heat treated at 673 K, (c) 873 K and (d) 1073 K.
R values of chips with no hydration treatment (a), with 10 min. (b), 30 min. (c), 60 min. (d) were 58%, 72%, 100%, 100%, respectively. With increase in the hydrides content of the chips, the collect rate also increased in milling. That is, the brittle TiH2 compounds formation in Ti-64 chips enhances the milling ability improvement, and fine powder can be produced via milling process. Figure 6 reveals the particle size distribution (a) and median particle diameter (b) of each hydration treated Ti-64 materials after 60 min. milling process. As mentioned above, Ti-64 chips with no hydration treatment and with 673 K treatment contain original spiral materials. Therefore, the particle size was measured after sieving these spiral chips. As shown in (a), the milled powders after no hydration nor 673 K hydration treatment had the almost same size distribution as well as the median particle diameter of 460 μm. This agreement between two different samples is because of the same hydrogen content (63~66 ppm) of both chips, and results in the similar mechanical milling behavior. When employing the hydration at 873 K and 1073 K, both milled Ti materials showed smaller particle size distributions. However, Fig. 6 (b) showed the median particle diameters of 119.1 μm and 189.2 μm for hydration treated materials at 873 K and 1073 K, respectively. SEM observation on Ti-64 milled powder after 1073 K hydration indicates that much fine milled particles with 1~5 μm diameter were agglomerated and a lot of coarse secondary particles were formed. A larger hydrogen content of 0.68 mass% (1073 K hydration treated chips) is effective for a milling ability of Ti-64 chips by larger amount of brittle TiH2 compounds, compared to the chips with 0.63 mass% hydrogen via 873 K hydration treatment. However, very fine milled Ti powders are easily agglomerated to form coarse powders. For example, in comparison of the cumulative frequency of coarse powder over 300 μm diameter, the milled materials via 873 K and 1073 K hydration treatment showed 6.1% and 19.4%, respectively. Therefore, this agglomeration between fine particles caused a larger median particle diameter of 189.2 μm when employing 1073 K heat treatment.

(a) Particle size distribution of 60 min ball-milled powder using Ti-64 chips heat treated at 673 K, 873 K and 1073 K, and (b) median particle diameter of each milled Ti-64 powder.
When 873 K hydration treatment was employed, the particle size distribution of the Ti-64 materials with milling for 10~60 min. was analyzed to investigate their mechanical milling behavior. As shown in Fig. 7, the distribution shifted to smaller particle diameter with increase in the milling time. The median values via 10 min, 30 min and 60 min milling process were 322.4 μm, 161.3 μm and 119.1 μm, respectively. In addition, the cumulative frequency of coarse particles over 300 μm were 54.6%, 17.8% and 6.1%, respectively. The above results mean that the fragmentation of coarse Ti-64 chips or particles occurred in a short time due to a significant milling ability improvement by brittle TiH2 compounds formation.

Particle size distribution of heat treated Ti-64 chips after ball milling at 10 min, 30 min and 60 min (heat treatment temperature; 873 K).
According to TG-DTA result in Fig. 2, the dehydration heat treatment at 873 K for 30 min was determined, and applied to the Ti-64 fine powder to remove hydrogen elements, where hydration temperature of 873 K and milling time of 60 min. were employed. It was clarified that hydrogen content was 0.007 mass% after dehydration treatment, which was the same level as the as-received Ti-64 raw chips. It was also satisfied with the hydrogen content regulated in JIS-60 (<0.015 mass%). That is, the following dehydration treatment at 873 K was effective to completely decompose the hydrides and remove the residual hydrogen of the milled Ti powder. Furthermore, the contents of oxygen; 0.06 mass%, nitrogen; 0.04 mass% and carbon; 0.04 mass% were completely satisfied with each element in JIS-60. It is concluded that the combination of the hydration, milling and dehydration process was effective and useful to directly reproduce Ti alloy powders used for PM components from their wasted spiral chips.
3.3 Compactibility and sinterability of reproduced Ti-64 powdersFive grams of the above Ti-64 reproduced powder via 60 min. milling and dehydration process (named as “recycled Ti-64”) was consolidated in 11.5 mm diameter die by applying 800 MPa at room temperature. The commercial Ti-64 alloy powder (ACA150: Toho Technical Service Co.) with a mean particle size of 106 μm was also pressed under the same conditions. The green compacts were sintered at 1273 K for 120 min. in vacuum, and the relative density of each sintered body was measured by Archimedes principle, where a true density of Ti-64 is 4.43 g/cm3. The calculated results of the relative density of green compacts and sintered materials are shown in Table 2. It indicates that a sinterability of the recycled Ti-64 powder is superior to the commercial one while its compactibility is slightly inferior to the commercial Ti-64 powder. This is because as mentioned in the previous studies16–18), the sintering behavior between recycled Ti-64 particles was accelerated by a diffusion of hydrogen atoms, which were originated from TiH2 compounds, along α-Ti grain boundaries. In addition, the hydrogen and oxygen contents of the sintered specimen using recycled Ti-64 powder were satisfied with JIS-60, that is, this technology has a significant possibility to be employed as a recycling process of machined Ti alloy chips in mass production.
| Green density [%] |
Sintered material | |||
|---|---|---|---|---|
| R.D.[%] | H[mass%] | O[mass%] | ||
| Recycled Ti-64 | 77.8 | 93.4 | 0.006 | 0.06 |
| Commercial Ti-64 | 80.5 | 91.1 | 0.002 | 0.29 |
In this study, the milling ability of coarse machined Ti alloy chips with spiral shape was significantly improved by formation of the brittle titanium hydrides (TiH2) in the chips via hydration heat treatment. In addition, the compactibility and sinterability of the recycled Ti alloy fine powder were also evaluated.
(1) According to TG-DTA result of hydrides formation behavior, the application of hydration heat treatment at 873 K or more was available to successfully synthesize TiH2 compounds in spiral Ti-64 chips. The fine Ti alloy powder with a median particle size of 120 μm was reproduced from the chips by 60 min mechanical milling process under 100% collect rate. Furthermore, the additional dehydration heat treatment completely decomposed the residual hydrides, and resulted in the hydrogen content was 0.007 mass%, which was the same as that of as-received Ti-64 original raw chips.
(2) The sintered Ti material using the above milled powder revealed a higher relative density compared to the commercial Ti-64 sintered body. At the same time, hydrogen and oxygen contents of the sintered specimen using recycled Ti-64 powder were satisfied with JIS-60. Accordingly, the machined Ti alloy chips were directly recycled as valuable starting materials to reproduce PM components via a combination of hydration-milling-dehydration process.
This work was partially supported by the Light Metal Educational Foundation.