2017 Volume 58 Issue 2 Pages 211-217
2017 Volume 58 Issue 2 Pages 211-217
The corrosion behavior and the amount of absorbed hydrogen in steel were investigated in neutral and alkaline solutions with pH values ranging from 8.3 to 12.4. The amount of absorbed hydrogen into steel during immersion in the solutions was evaluated by thermal desorption analysis. In the alkaline solution of pH 12.4, the steel maintained a noble potential in a passive state, and almost no hydrogen absorption into the steel was detected. However, as the pH moved towards a more neutral pH, the corrosion potential shifted in the less noble direction, and the amount of hydrogen absorbed increased dramatically. These results indicate that the steel surface became more active in the neutral solutions, and the hydrogen evolution reaction, one of the cathodic reactions of steel corrosion, was enhanced close to the neutral pH with decreasing corrosion potential in the less noble direction. The change of the surface state from passive to active with decreasing pH accelerated the anodic dissolution of steel and made the corrosion potential less noble, resulting in the enhancement of hydrogen evolution and absorption reactions on the steel.
Pre-stressed concrete (PC) is a structural material with high strength and resiliency to both tensile and compressive stresses. It has therefore been used for poles, slabs, and beams in a range of infrastructure applications. To introduce compressive stress to concrete structures, pre-stretched high-strength steels are often embedded in concrete slurry. In addition to strengthening of PC, formation of cracks is mitigated due to compressive stress in concrete structures. These advantages elongate the lifetimes of concrete structures in usage environments.
However, over many years, cracks form in PC by aging degradation of the concrete. One aging degradation mechanism in concrete structures is carbonation, where carbon dioxide in the atmosphere reacts with calcium in concrete, resulting in a lower pH, i.e., neutralization1,2). After the formation of cracks, uptake of waters containing corrosive agents occurs; these waters reach the surface of the pre-stressed steels in the PC. Normally, steels in concrete structures are in a passive state due to the formation of a passive film in high-alkaline concrete environments3). However, aging degradation of concrete changes the solution chemistry of the pore water in cracks in concrete structures due to carbonation and uptake of Cl ions, resulting in a lower water pH and depassivation of steel. Therefore, steel corrosion occurs depending on the extent of aging.
In an aqueous environment, hydrogen atoms can evolve through a cathodic reduction reaction, as described by eqs. (1) and (2), depending on the pH of the environment. These reduction reactions are one of a number of cathodic reactions that occur during metallic corrosion. The evolved hydrogen atoms are adsorbed onto the surface of metallic materials through the reaction shown in eq. (3). Then some amount of the adsorbed hydrogen is absorbed into metallic materials through hydrogen adsorption processes4,5):
| \[{\rm H^+ +e^- } \to {\rm H_{ad}}\] | (1) |
| \[{\rm H_2 O +e^- } \to {\rm H_{ad} +OH^- }\] | (2) |
| \[{\rm M + H_{ad}} \to {\rm M - H_{ad}}\] | (3) |
However, according to some reports, cracking or failure of steels in concrete structures occurs even in alkaline environments8,9), where cracking or failure is thought to be associated with hydrogen embrittlement of steels. Hydrogen embrittlement is one environmental deterioration phenomenon in which metals and alloys suddenly break down due to absorption of hydrogen atoms under applied tensile stress. In addition, high-strength steels used in PC to apply high compressive stress to concrete structures have a relatively higher susceptibility to hydrogen embrittlement due to their high strength. Therefore, to investigate the potential for hydrogen embrittlement in aging concretes, the hydrogen absorption behavior of steels should be studied in neutral and weak alkaline solutions. However, the mechanism and quantity of hydrogen absorption into steels in neutral and weak alkaline solutions have not been reported and are not yet fully understood.
In this study, hydrogen absorption into steels in neutral and weak alkaline solutions simulating pore water in aging concretes is investigated. In addition, the possibility of environmental degradation of steels in neutral and weak alkaline solutions is discussed based on the corrosion behavior and the amount of absorbed hydrogen.
The material used in this study was a 1420-MPa-class high-strength steel bar with a diameter of 9 mm. The chemical composition of the steel bar is shown in Table 1. The surface of the as-received steel bar was machined to remove surface scales (black skin) formed during the steelmaking process. The steel bar was sliced into small disks with a thickness of about 3 mm. The surface of the steel disks was ground successively with SiC paper down to a Japan Industrial Standard (JIS) #800 grit. Finally, the steel disks were rinsed ultrasonically in acetone and used as samples for electrochemical and corrosion tests.
| C | Si | Mn | S | P | Fe |
|---|---|---|---|---|---|
| 0.34 | 0.27 | 0.78 | 0.006 | 0.017 | Balance |
A series of test solutions, listed in Table 2, were prepared with reagent grade chemicals (Kanto Chemical Co., Inc., Japan) and deionized water. The pH of the test solutions was adjusted from 8.3 to 12.4 to simulate the pH of pore waters corresponding to the extent of neutralization in aging concretes. One test solution was an alkaline solution of 50 mol/m3 NaOH at pH 12.4, to simulate pore water in fresh concrete. The other test solutions were prepared across a pH range from 8.3 to 11.1 by adding Na2CO3 and NaHCO3 to an NaOH solution, while maintaining the total carbonate concentration at 50 mol/m3. All test solutions also contained 1 kmol/m3 Na2SO4 as a supporting electrolyte.
| No. | Test solution | pH |
|---|---|---|
| 1 | 50 mol/m3 NaOH + 1 kmol/m3 Na2SO4 | 12.4 |
| 2 | 50 mol/m3 Na2CO3 + 1 kmol/m3 Na2SO4 | 11.1 |
| 3 | 25 mol/m3 NaHCO3 + 25 mol/m3 Na2CO3 + 1 kmol/m3 Na2SO4 | 9.8 |
| 4 | 45 mol/m3 NaHCO3 + 5 mol/m3 Na2CO3 + 1 kmol/m3 Na2SO4 | 9.3 |
| 5 | 50 mol/m3 NaHCO3 + 1 kmol/m3 Na2SO4 | 8.3 |
Immersion and electrochemical polarization tests were undertaken in a conventional three-electrode cell with an HZ-5000 potentio/galvanostat (Hokuto Denko Corp., Japan) to investigate the corrosion behaviors of the steel disks in the test solutions. One steel disk sample served as the working electrode for each test. A silver/silver chloride electrode (SSE) in saturated KCl and a platinum sheet were used as the reference electrode and the counter electrode, respectively.
In the immersion tests, the corrosion potential of the steel disks was monitored for 24 h in the test solutions. For measurement of dynamic polarization curves, the steel disks were polarized from a corrosion potential in the anodic or cathodic direction at a scan rate of 0.5 mV/s. These measurements were undertaken at an ambient temperature of about 25℃.
2.3.2 Surface analysisThe surface of the steel disks after the immersion tests was observed with a JSM-6010LA scanning electron microscope (JEOL Ltd., Japan). Corrosion products formed on the steel disks during the immersion tests were analyzed with a MiniFlex600 X-ray diffractometer (Rigaku Co., Ltd, Japan). X-ray diffraction measurements were undertaken using the θ-2θ method at 40 kV and 15 mA with a wavelength of 0.15418 nm (Cu-Kα).
2.3.3 Thermal desorption analysisHydrogen desorption rates from steel disks after 72-h immersion in the test solutions were measured by thermal desorption analysis (TDA) with a TDS1200 (ESCO Co., Ltd., Japan). The steel disks (diameter: 9 mm; thickness: 3 mm) were used as samples. The TDA measurements were undertaken within 30 min of immersion test completion to minimize the release of absorbed hydrogen from the steel disks at room temperature. After the immersion tests, the steel disks were set in a vacuum chamber at a pressure of the order of 10−7 Pa and heated from room temperature to 500℃ at a constant heating rate of 200℃/h. The TDA spectrum for the steel disks without the immersion test was also obtained as a reference.
Figure 1 shows changes in the corrosion potential of steel disks during 24-h immersion in test solutions over a range of pH values. In each test solution, the corrosion potential decreased rapidly in the less noble direction just after immersion, and then reached an almost constant value. It can also be seen from Fig. 1 that the corrosion potential becomes less noble in solutions with decreasing pH.
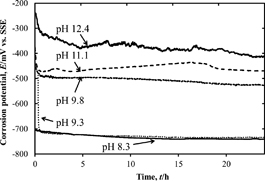
Changes in the corrosion potential of steel disks during 24-h immersion in test solutions over a range of pH values.
The corrosion potential after 24-h immersion is plotted as a function of pH in Fig. 2. In the higher pH region above 9.8, the corrosion potential increased with pH (slope: 55 mV/pH). In contrast, in the pH region below 9.8, the corrosion potential changed significantly, by more than 200 mV, in the less noble direction. The corrosion potential did not change significantly in the pH region below 9.3, regardless of the pH value.

Change in corrosion potential for the steel disks after 24-h immersion tests as a function of solution pH.
Figure 3 shows SEM images for the surfaces of the steel disks after the 24-h immersion tests; steel surfaces exposed to a pH higher than 11.1 retained a metallic luster slightly, although at pH 11.1 some iron corrosion products were observed on the steel surface. In contrast, the surfaces of the steel disks exposed to a pH lower than 9.8 were obviously covered in iron corrosion products, and the amount of the corrosion products increased with decreasing pH (Fig. 3). Further, as shown in Fig. 4, FeOOH and/or Fe2O3 were detected by X-ray diffraction measurements on steel after immersion in the solutions with a pH less than 9.8. These results indicate that the steel surface is in a passive state in the pH 12.4 solution, but that the passive state cannot be maintained after immersion in a solution lower than pH 9.8.

SEM images for the surfaces of the steel disks after the 24-h immersion tests with various pH values: (a) pH 8.3, (b) pH 9.3, (c) pH 9.8, (d) pH 11.1, and (e) pH 12.4.

XRD patterns for the surfaces of the steel disks measured after 24-h immersion tests in various pH solutions.
For further investigation of the corrosion behavior, anodic and cathodic polarization curves for steel disks were measured in the test solutions with various pH values. Figures 5 and 6 show the anodic and cathodic polarization curves for the steel disks, respectively. The anodic polarization curves shifted in the anodic direction with increasing pH, as shown in Fig. 5. Furthermore, the anodic current densities also decreased sharply at pH between 9.3 and 9.8. In the test solution at pH 12.4, passive behavior was clearly visible around the corrosion potential. These anodic polarization behaviors at various pH values are in good agreement with the surface observations and XRD results shown in Figs. 3 and 4.

Anodic polarization curves for the steel disks measured in the test solutions with various pH values.
The cathodic polarization behaviors of the steel disks in the test solutions also depended on pH, as shown in Fig. 6. In the case of a pH higher than 9.8, a diffusion-limiting current density due to the reduction of dissolved oxygen was observed; the current increased with cathodic polarization, due to the hydrogen evolution reaction. In contrast, in the case of a pH lower than 9.3, a limiting current density was not clearly visible. The difference between these cathodic polarization behaviors at different pH values is due to the difference in the magnitude of anodic polarization behavior around the corrosion potential. Figure 5 shows a Tafel plot of the anodic current density for the steel disks in the test solutions with pHs of 8.3 and 9.3; the current density increased linearly with the anodic polarization from the corrosion potential. Therefore, the corrosion potential shifts in the less noble direction with decreasing test solution pH, and the diffusion limiting current region can be hindered with a high anodic current around the corrosion potential.

Cathodic polarization curves for the steel disks measured in the test solutions with various pH values.
From the results of the polarization measurements, polarization resistance for steel disks in the test solutions, which can be an index of corrosion rate, was calculated using the current-potential relationship around the corrosion potential. Figure 7 shows changes in the polarization resistance of the steel disks as a function of solution pH. Polarization resistances in the pH region below 11.1 increased slightly with pH (Fig. 7); however, the resistance increased dramatically at pH 12.4. These results suggest that the corrosion rate slows with increasing pH and that, in addition, at pH 12.4, the corrosion rate is quite low due to the passive state on the surface of the steel disks. These results are in good agreement with the surface morphology and electrochemical behavior shown in Figs. 5 and 6.

Change in the polarization resistance of the steel disks as a function of solution pH.
Figure 8 shows the TDA spectra for steel disks measured after immersion over a range of durations in the pH 8.3 test solution. The TDA spectrum labelled “Blank” was obtained without an immersion test. All spectra in Fig. 8 show that the peak hydrogen desorption rate is observed at almost the same temperature, meaning that the trapping state of absorbed hydrogen is nearly identical, regardless of immersion. Hydrogen atoms that are desorbed from the steel disks at room temperature are considered to be weakly trapped, so-called diffusible hydrogen10).

TDA spectra for steel disks measured after immersion over a range of durations in the pH 8.3 test solution.
Furthermore, as shown in Fig. 8, the peak heights of the hydrogen desorption rate increased steadily with immersion duration; thus, the amount of absorbed hydrogen increases with progressing steel corrosion. The TDA results indicated that hydrogen atoms evolve during steel corrosion and are absorbed by the steel disks in the pH 8.3 test solution.
The amount of absorbed hydrogen can be calculated by integrating the hydrogen desorption rate along the temperature axis from 30℃ to 200℃, because the temperature axis can be converted to time using the constant heating rate of 200℃/h. However, at this time, it is needed to take account of residual amount of absorbed hydrogen in steel disk before immersion tests. This is because the desorption of hydrogen atoms was detected on steel disks without an immersion test as observed in the spectrum labelled “Blank” in Fig. 8. The absorbed hydrogen in the steel disk before immersion test can be attributed to hydrogen evolution reaction occurring with steel corrosion during sample preparation such as slicing or polishing in aqueous media. In fact, hydrogen atom desorption was not detected from the steel disk that had been left in ambient environment for one week after sample preparation, because diffusible hydrogen was desorbed from the disks. Therefore, the residual amount of absorbed hydrogen calculated from the “Blank” TDA spectrum was subtracted from the calculated amount of absorbed hydrogen during the immersion tests.
Figure 9 shows the amount of absorbed hydrogen in the steel disks (the hydrogen content) as a function of immersion time in the pH 8.3 test solution. The hydrogen content increased rapidly with immersion time and saturated within 24 h.

Change in hydrogen content in the steel disks as a function of immersion time in the pH 8.3 test solution.
Figure 10 shows TDA spectra for the steel disks after 24-h immersion tests in test solutions over a range of pH values. The desorption of hydrogen atoms from the steel disks was detected for all pH cases, although for pHs 9.8 and 11.1, the peaks in the TDA spectra were small and nearly identical to the peak in the “Blank” spectrum (Fig. 10). This indicates that hydrogen atoms may not be absorbed during the immersion tests at pHs 9.8 and 11.1. Hydrogen atoms are absorbed into steel disks when the pH is less than 9.3.

TDA spectra for the steel disks measured after 24-h immersion tests in test solutions over a range of pH values.
Figure 11 shows the hydrogen content absorbed into the steel disks after 24-h immersion tests as a function of the pH of the test solutions. The hydrogen contents were also calculated using the same procedure as described before. The hydrogen contents for test solutions of pH greater than 9.8 were the same as for the “Blank” case; that is, few hydrogen atoms were absorbed into the steel disks during immersion in a pH over 9.8. In contrast, as shown in Fig. 11, the amount of absorbed hydrogen increased with decreasing pH below 9.3, indicating that hydrogen atoms are absorbed at pH values lower than 9.3.

Change in hydrogen content absorbed into the steel disks after 24-h immersion tests as a function of the pH of the test solutions.
The corrosion potentials of steel disks decrease significantly between pH 9.8 and pH 9.3 (Fig. 2). In addition, from corrosion morphology results, iron corrosion products clearly precipitated on the steel surfaces in test solutions with a pH less than 9.8. These results suggest that corrosion reactions taking place on steel surfaces change drastically at around neutral pH. These results coincide with those by Heut et al., who reported a transition from passive to active corrosion of steel in carbonated concrete at pH between 9.4 and 1011).
Similarly, as reported by Davies et al., carbon steel forms a pseudo-passive Fe(OH)2 and/or FeCO3 layer during anodic polarization in bicarbonate solution through the following reactions12,13):
| \[{\rm Fe+2H_2 O} \to {\rm Fe(OH)_2 + 2H^+ +2e^- }\] | (4) |
| \[{\rm Fe + HCO_3^-} \to {\rm FeCO_3 + H^+ + 2e^- }\] | (5) |
In this study, the fully passive state was observed at pH 12.4. On the other hand, although some parts of the surfaces retained metallic luster, small amount of corrosion products were observed on the steel disk tested at 11.1, as shown in Fig. 3. In addition, from the results of XRD measurements (Fig. 4), corrosion products of FeOOH and Fe2O3 were detected on steel after immersion in solutions with a pH less than 9.8. Thus, the passive state is broken at pH 11.1, leaving a pseudo-passive state. Further, with decreasing pH to neutral values, the dissolution of FeCO3, shown in eq. (6)2), takes place due to enhancement of the anodic reaction.
| \[{\rm FeCO_3 (aq) + 2H_2 O} \leftrightarrow {\rm FeOOH +2H^+ + e^- +HCO_3^- }\] | (6) |
In alkaline environments such as fresh concrete structures, the cathodic reaction taking place on steel is mainly the reduction reaction of dissolved oxygen2). However, in the case of neutral environments such as aging concretes, simultaneous reduction of hydrogen ions can also take place to form adsorbed hydrogen atoms on the steel surface. Furthermore, the adsorbed hydrogen atoms can be partially absorbed into the steel. Proverbio et al. reported that, even at pH 8.3, the hydrogen evolution reaction occurs on the steel surface with a potential lower than −730 mV vs. SCE, a calomel reference electrode in saturated KCl15).
The relationship between the hydrogen content and the corrosion potential of steel disks in test solutions with a range of pH values is shown in Fig. 12. The hydrogen content increased dramatically when the corrosion potential was less than −600 mV vs. SSE. This result suggests that adsorption of hydrogen atoms occurs in test solutions of pHs 8.3 and 9.3 due to the reduction reaction of water. The slight decrease in the charge transfer resistance in the test solutions with pHs lower than 9.8 suggests that hydrogen ions can be reduced simultaneously. This result is also in good agreement with the polarization behaviors shown in Figs. 5 and 6. Therefore, hydrogen atoms are absorbed in steel due to a decrease in the corrosion potential.

Relationship between the hydrogen content and the corrosion potential of steel disks in test solutions with a range of pH values.
To investigate whether hydrogen evolution can occur thermodynamically during the immersion tests, corrosion potentials measured after 24-h immersion were plotted in a pH-potential diagram for iron at 25℃ in the aqueous solutions containing carbonates (Fig. 13). In this diagram, the total concentrations of iron and carbonate species were maintained at molarities of 1 × 10−6 mol/kg and 50 mmol/kg, respectively. The corrosion potentials in the solution at pH 9.3 were nearly on the equilibrium line for hydrogen evolution. In addition, the corrosion potential is less noble than the line at pH 8.3. These results support the conclusion that hydrogen evolution takes place on steel disks during immersion in test solutions with a pH of less than 9.3.
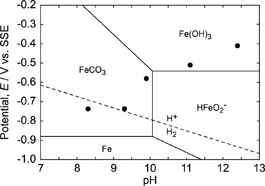
Potential-pH diagram for iron in neutral and alkaline solutions containing CO32− ions.
The process of hydrogen absorption into steel in neutral and weak alkaline solutions simulating pore water in aging concretes can be summarized as follows. At the beginning of the exposure of fresh concretes to atmospheric corrosion environments, steels are in high-alkaline pore water. Under these conditions, the steels are in a passive state and maintain higher corrosion potentials than the line of hydrogen evolution, resulting in no hydrogen atom absorption into steels. However, as the pH in the pore water decreases due to aging and neutralization, the surface state of the steels changes from the passive to the pseudo-passive or active state. This transition can enhance steel corrosion, such that corrosion potentials shift to less noble ones. However, few hydrogen atoms are absorbed into the steel due to the high corrosion potential, which is maintained by the formation of a pseudo-passive film. Then, as concrete is carbonated further, the anodic reaction transitions occur from the formation of a pseudo-passive film to iron dissolution. The corrosion potential decreases at pH values below pH 9.3 due to anode reaction acceleration. As a result, the corrosion potential decreases significantly and hydrogen is absorbed into the steel.
As summarized by Matsuyama, the susceptibility to hydrogen embrittlement of high-strength steels is mainly associated with their strength16). In this study, 1420-MPa-class steels were used as the test material. According to the schematic drawing of the relationship between the susceptibility for hydrogen embrittlement and the strength of steels, a concentration of diffusible hydrogen atoms at the sub-ppm level can be considered to have potential for hydrogen embrittlement for 1500-MPa-class steels. Although the hydrogen content evaluated at pH 8.3 is one-order lower than the sub-ppm level, as shown in Fig. 12, the hydrogen content can increase locally at defects in the steel, such as vacancies and dislocations17,18). Therefore, to investigate further whether hydrogen embrittlement can occur in aging concretes, the mechanical properties of steel after immersion tests in neutral solutions should be evaluated.
Hydrogen absorption behavior into steel was investigated using corrosion and electrochemical measurements and thermal desorption analysis in neutral and weak alkaline solutions simulating pore waters in aging concrete. The findings were as follows: