2017 Volume 58 Issue 2 Pages 127-130
2017 Volume 58 Issue 2 Pages 127-130
In this study, we were able to form strong Au-to-Au joints using a silver oxide (Ag2O) paste sintered at 300℃. We successfully reduced the processing pressure applied during sintering by 0.5 MPa by optimizing the sintering conditions. Through X-ray diffraction phase analysis as well as crystallite size calculations, we observed a significant reduction (27%) in the crystallite size as the heating rate was increased from 10℃/min to 180℃/min. Scanning electron microscopy cross-sectional observations confirmed that the high heating rate facilitated sintering within the bonding layer. The bonded shear strength (maximum of 24 MPa) was higher than that of the conventional Pb-5Sn solder (18 MPa), proving the suitability of Ag2O paste as a potential lead-free bonding material for high-temperature applications.
The on-going rapid developments in high-performance electronic devices have led to such devices being used under thermally severe environments. To be able to withstand such conditions, not only do the electronic components have to be durable but the bonding and electronic connections need to be stable as well. Traditionally, high-melting-point solders, including Pb-5Sn and Pb-10Sn, had been used for such purposes; however, these solders contain lead, which has been banned owing to its health concerns. Various materials have been proposed for replacing these high-melting-point solders. Studies exploring the use of nanoparticles as a bonding material have shown that they have great potential in terms of the joint shear strength1–6). Furthermore, we have successfully used Ag2O as a bonding material, replacing the nanoparticles as the original material and thus filling the cost gap between conventional solders and nanoparticle-based materials7–15). In this process, a bonding paste containing Ag2O and a reducing solvent is used as the solder material, generating Ag nanoparticles in-situ during the heating process. Bonding is achieved as the generated nanoparticles undergo sintering and form a bonding layer. The sintering happens at a relatively low temperature owing to the high surface energy of the nanoparticles, which allows for the thermal pressure applied on the electronic devices being soldered to be minimized.
One of the remaining concerns regarding this bonding process using a Ag2O paste is the process pressure during the heating stage, which results in the formation of a coarse bonding layer, allowing for the evacuation of gases as they are generated during the reduction reaction. In order to reduce the mechanical stress experienced by the electronic components upon bonding, the process pressure needs to be minimized. However, the presence of this pressure is one of the essential driving forces for the sintering of the nanoparticles, and a lack of pressure could lead to an inhomogeneous bonding layer with numerous voids and vacancies.
To compensate for the lack of the process pressure during bonding, we focused on the optimization of the other process parameters, such as the sintering temperature and heating rate. In terms of the heating rate, the rapid firing of fine particles is known to be advantageous with respect to their densification at relatively low temperatures16). Further, fast firing is beneficial in that it brings the material directly to higher temperatures, making it possible to achieve a high density in the case of fine grains while avoiding low temperatures. This method has been used with many systems, including the Al2O317), ZrO216), and Y2O318) ones. However, it has not yet been exploited for electronic bonding and neither has it been used with systems that undergo chemical reactions during the firing process. In this paper, we present a low-pressure bonding process based on the use of Ag2O paste and its process control while focusing on the optimization of the heating rate. We investigated how changes in the heating rate affect the reduction reaction mechanism as well as the resulting sintered structures and explored ways of applying this method for Au-to-Au bonding.
We prepared the Ag2O paste using granulated Ag2O and a reducing solvent. Ag2O particles (Japan Pure Chemical) were processed using a mortar (made of alumina) and mixed with diethylene glycol (Tokyo Chemical Industries). The amount of solvent used with the oxide powder was optimized at 150 μl/g. The paste was mixed in a centrifuge for 5 min.
The bonding substrate was copper coated with nickel (thickness of approximately 2 μm)/gold (thickness of approximately 0.7 μm)3,6,8,12). For bonding, the paste was placed on the bottom substrate (10 mm in diameter and 5 mm in length) using a 50-μm-thick stainless mask. The specimen was then preheated in a furnace for 5 min at 100℃, in order to eliminate the redundant solvent within the printed paste. After the preheating treatment, the upper substrate 5 mm in diameter and 2 mm in length to be bonded was placed on top of the dried Ag2O paste layer, and the specimen pair was placed in an infrared furnace for bonding at a pressure of 0.5 MPa in air. The bonded specimens were cooled in air to room temperature without using any cooling apparatus.
To determine the basic thermal characteristics of the Ag2O paste, differential thermal analysis (DTA) (DSC8230, Rigaku) and thermal gravimetry (TG) (TG8120, Rigaku) measurements were performed in air. For microscopic observations, we used a scanning electron microscopy (SEM) (S-4800, Hitachi High Technologies) system as well as X-ray diffraction (XRD) (PW3040/60X'Pert Pro, Philips) system. Shear strength tests were carried out using an Autograph (DCS-10T, Shimadzu) system at a cross-head speed of 30 mm/min.
Figure 1 shows the TG/DTA curves of the Ag2O paste when heated at the rates of 10℃/min and 100℃/min. The positions of the peaks indicate the formation of Ag through a redox reaction between the Ag2O paste and the reducing solvent. The peak shift at the weight-loss temperature indicates that the reduction temperature increased by 38℃ as the heating rate was increased from 10℃/min to 100℃/min. In addition, while the weight loss occurred over an extended range of temperatures when the heating rate was 10℃/min, in the case of the rate of 100℃/min, the loss occurred immediately after the reaction had started. This can be attributed to the difference in the induced heat flow (see Fig. 1). The increased heating rate resulted in the generation of a 1.5 times larger amount of heat during the endothermic reduction reaction.
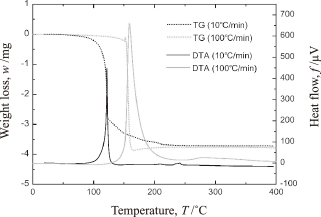
Results of the TG/DTA analysis of the Ag2O paste for a heating rate of 10℃/min.
In order to further investigate this phenomenon during the bonding process, we performed XRD analyses on the Ag2O paste at several points during the heating process, in order to determine the temperature at which the reduction reaction occurs. The paste was placed onto the specimen to be bonded and the same heating procedure was performed until the temperature had reached the desired level. Once this had happened, we immediately removed the bonded specimen, prepared the sample for the XRD analysis, and measured the diffraction patterns. The patterns are shown in Figs. 2 and 3. We used two different heating rates, namely, 10℃/min (Fig. 2) and 180℃/min (Fig. 3), in order to elucidate the effects of the heating process. It can be seen from Fig. 2 that the reduction of the Ag2O paste to Ag nanoparticles had occurred to completion before the temperature reached 200℃, as the Ag2O peak had disappeared. On the other hand, the Ag2O peak was visible till 300℃ when the heating rate was set to 180℃/min (Fig. 3). This proved that an increase in the heating rate retarded the reduction reaction. In other words, the generation of Ag nanoparticles was delayed and occurred at a higher temperature.

XRD patterns of the Ag2O paste obtained at temperatures of (a) 150℃ and (b) 200℃ when heated at the rate of 10℃/min.
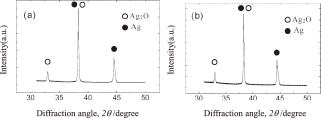
XRD patterns of the Ag2O paste obtained at temperatures of (a) 250℃ and (b) 300℃ when heated at the rate of 180℃/min.
We then investigated the crystallite growth mechanism using the XRD patterns, in order to better understand the effects of the bonding processes on the sintering behavior of Ag nanoparticles. Figure 4 shows the size of the Ag crystallites as a function of the temperature for the two different heating rates (10℃/min and 180℃/min). Here, the crystallite size was calculated using the Scherrer equation:
| \[D = \frac{{\rm K}\lambda}{B\cos\theta}\] | (1) |
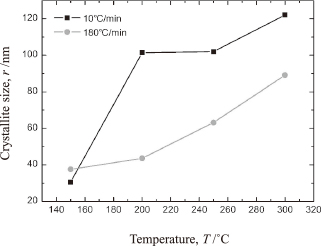
Size of the Ag crystallites as a function of the sintering temperature. The samples were removed immediately from the sintering furnace once they had reached the desired temperature and their crystallite sizes were determined via XRD measurements.
In this bonding process, sintering can occur at a temperature lower than 300℃ only when Ag exists in the form of nanoparticles, as their significantly high surface energy results in necking and subsequent agglomeration, causing relaxation and the minimization of their energy5,8,10). It can be concluded that quick firing is advantageous in that it allows one to bypass the low-temperature regime, resulting in an increase in the surface energy at higher temperatures. It is known that, at higher temperatures, densification occurs preferentially instead of grain growth, owing to surface diffusion21). The other benefit is that the phenomena of necking and wetting favor higher sintering temperatures because the heat lowers the surface tension of the bonding material22). Last but not the least, the capillary force of the residual reducing solvent is one of the strongest attractive forces acting on gathering the generated nanoparticles during the Ag2O paste bonding process6). It is critical to fully leverage these driving forces (i.e., the surface energy and sintering temperature) to achieve robust bonding when a limited mechanical process pressure is applied.
SEM images of the cross-section of the bonded layer are shown in Fig. 5. The bonding specimens were kept at 300℃ for 30 min to allow for bonding and were then processed to prepare the sample for the cross-sectional observations. It is apparent that a higher heating rate resulted in better wetting and void elimination, even in a system in which a redox reaction took place. We could confirm that the higher heating rate resulted in two advantages: grain growth and the densification of the resulting sintered layer. As these processes benefit from diffusion, it could be concluded that increasing the heating rate to reach a higher temperature was effective in driving diffusion and achieving a dense, sintered structure23,24).

SEM micrographs of the cross-section of the bonded areas after the sintering process for heating rates of: (a) 10℃/min and (b) 180℃/min.
Figure 6 shows the changes in the shear strength of the bonded joints as a function of the holding time at 300℃. When the temperature reached 300℃ (t = 0 min), despite its randomness, the joint bonded at a heating rate of 10℃/min was stronger than that bonded at 180℃/min; this can be attributed to the crystallite growth behavior shown in Fig. 4. After being held for 5 min, however, the strength of the joint formed at 180℃/min began to increase. After being held at 300℃ for 30 min, the joint exhibited a shear strength of 24 MPa, which is higher than that of joints bonded using conventional high-temperature solders (18 MPa)2). Interestingly, the strength of the joint bonded at 10℃/min did not increase throughout the holding process. This phenomenon can also be explained by Fig. 4, which shows that the crystallite growth rate decreased even as the operating temperature was increased. It can be argued that the high surface energy of the generated Ag nanoparticles resulted in proactive surface diffusion from the start of the heating process, which was before the temperature-driven densification caused by the lowered surface tension of the bonding materials. A higher heating rate allowed the high surface energy to be maintained until the operating temperature had reached 300℃ or even till the reduction reaction occurred, once the temperature had reached 300℃. Once the Ag nanoparticles had been generated, they immediately began agglomerating, driven by their surface energy as well as by the decrease in their surface tension. It can thus be concluded that rapid heating during the Ag2O paste bonding process led to an effective reduction reaction as well as rapid densification within the bonding layer. These two phenomena in combination are what resulted in the proposed method being suitable for forming strong joints under limited process pressure.
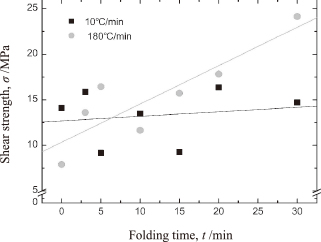
Shear strength as a function of the holding time at 300℃.
This study was financially supported by a Grant-in-Aid for Scientific Research (B) (No. 26289248) from the Japan Society for the Promotion of Science.