2017 Volume 58 Issue 3 Pages 423-426
2017 Volume 58 Issue 3 Pages 423-426
The poor plasticity of amorphous metal at room temperature has proved to be one of the biggest obstacles for its widespread application as structural components. In present study, the experimental samples were prepared by suction-casting and high vacuum die-casting with Zr54.6Ti13.8Cu9.3Ni5.8Be13.5Nb3 (at. %) to discuss the effect of the oxygen content and cooling rate on the plasticity of amorphous metal respectively. The results indicate that dendrite with appropriate dimension and fraction can be obtained by adjusting oxygen content so as to improve the plasticity of metallic glasses. Likewise, the appropriate cooling rate even above the critical cooling rate can also be quite effective to make the brittle metallic glasses obtain high plasticity.
Amorphous alloys, also known as metallic glasses, with special atomic arrangement inherited directly from the liquid state, did not emerged until 1960, when Duwze et al.1) prepared the glassy Au-Si alloy by means of quenching the molten metal. Since 1980's, the research group of A. Inoue2–4) in Tohoku University and W.L. Johnson et al.5,6) in California Institute of Technology developed a series of multi-element amorphous alloys with large size. Their critical cooling rate for glass formation is dramatically reduced and the qualitative leap of critical dimension makes these alloys genuinely bulk metallic glasses (BMGs)7,8). All of these work provided a strong foundation for the engineering application of amorphous alloys.
Compared with conventional polycrystalline metallic materials, metallic glasses have many excellent mechanical properties, such as high fracture toughness, distinctly higher tensile strength, much lower Young's modulus, outstanding super-plasticity at their supercooled regions9–11). Unlike crystalline alloys, metallic glasses do not have dislocation defects resulted in yielding and plasticity during the deformation process and its plastic deformation is localized or restricted in one shear bands where the deformation becomes so large that it is physically broken suddenly. Usually the compression plastic strain is not more than 2% and tensile plastic strain at room temperature is almost zero12). The poor plasticity increases the scattering tendency of mechanical properties which leads to catastrophic failure of BMGs without enough reliability. That has seriously restricted the application of amorphous metals as structural materials in engineering13,14). However, in 2007, W.H. Wang's research group prepared ZrCuNiAl amorphous metal with high strength (>1.7 GPa) and plasticity (elastic strain is only 2% and true strains is about 160%) at room temperature through the appropriate composition choice15). Since then, the plasticity of amorphous metal becomes the research focus again as one of the core scientific questions in amorphous physics and material field.
In recent years intensive efforts have been made to develop many promising approaches to toughen BMGs which can be divided into two categories. One is the extrinsic toughening by surface treatment, pre-deformation or developing a composite microstructure with external ductile phase dispersed within the glassy matrix16–24). Another is the intrinsic toughening by designing phase separation, increasing the Poisson's ratio or the amount of free volume in BMGs, adjusting the Young's modulus and so on25–29). In generally, the latter approach can be implemented in technique through tailoring composition, changing cooling rate, thermomechanical treatment, etc. In author's opinion, the latter approach should be a more ideal solution because the brittleness of BMGs can be solved in nature. However technological means have not been explored broadly and in depth to fulfill the latter approach30). In the present paper, the effect of oxygen content and cooling rate on the plasticity of Zr54.6Ti13.8Cu9.3Ni5.8Be13.5Nb3 BMGs will be discussed qualitatively. Our aim is to provide some feasible methods for improving the plasticity of amorphous metal.
The master alloy ingots with a nominal composition of Zr54.6Ti13.8Cu9.3Ni5.8Be13.5Nb3 (at.%) were prepared by arc-melting mixtures of Cu(99.99%), Zr(99.99%), Ti(99.99%), Ni(99.9%), Nb(99.99%) and Be (impurity<0.01%). The ingots were up-side down and remelted three times in order to ensure chemical homogeneity. Two kinds of ingots with different oxygen content were prepared by means of adjusting the base pressure of vacuum chamber, 3 × 10−4 Pa and 3 × 10−2 Pa respectively. And then the ingots were remelted and suction-casted into a copper mold to obtain the plate specimens (Fig. 1(a)) with dimensions of 100 × 10 × 2 mm3. In order to investigate the effect of cooling rate on the mechanical properties of BMGs, the specimens with different thickness (0.8, 1.0, 1.2 and 1.6 mm) were prepared by using the ingots with low oxygen content. Vacuum die-casting method was applied to prepare these samples because it is difficult to manufacture thin specimen whose thickness is less than 2 mm by common suction-casting or melt spinning methods. The typical as-cast plate sample without cutting the biscuit is shown in Fig. 1(b)31).

Two kinds of experimental samples prepared by (a) suction-casting and (b) high vacuum die-casting.
Sans ETM503C testing device was used for three-point bending tests at a constant strain rate 0.001 s−1. NCS IRO-II oxygen and nitrogen analyzer with 1 ppm measurement accuracy was used to measure the oxygen content of samples. An optical microscopy Leica DM2500P was used to observe the morphology of crystalline phase. A SHIMADZU LabX XRD-6000 X-ray diffraction with Cu Kα radiation was used to examine the crystalline structure. Transmission electron microscopy (TEM) specimens were dimpled using a Gatan 656 dimpler with 1 μm diamond abrasive and the polishing wheel until the thickness of central region was less than 5 μm. A Precision Ion Polishing System (GatanModel 691) with a liquid-nitrogen cooling system was used for final polishing using both argon ion beams at 2.5 keV and ±4 inclination to the specimen surface. The detailed microstructures were investigated by high-resolution transmission electron microscopy (HRTEM; JEOL, JEM-2010F) combined with energy dispersive X-ray spectrometry (EDX; Oxford instrument INCA system).
Table 1 shows the oxygen content of 1# and 2# samples prepared by suction-casting method. The oxygen content of each sample was measured three times and the average value was calculated so as to reduce error effectively. It is obvious that the oxygen content of 1# sample (9070.1 ppm) is an order of magnitude larger than that of 2# sample (927.8 ppm). Therefore the change of base pressure of vacuum chamber in the preparation process can effectively adjust the oxygen content of sample and achieve our experimental design.
| Sample number | Oxygen content (ppm) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Average value | |
| 1# | 9327.6 | 9039.2 | 8843.5 | 9070.1 |
| 2# | 911.3 | 912.3 | 959.8 | 927.8 |
Figure 2(a) presents the stress-strain curve of samples 1# and 2# through three-point bending tests. Compared to the little plasticity characteristics of sample 1#, sample 2# exhibits considerable plasticity and its strength is also increased by 17.4% (the strength of sample 1# and 2# is 1805 MPa and 2119 MPa respectively). The whole preparation process of both samples is almost same except for base pressure of vacuum chamber which leads to different oxygen content of the samples. In other words, the different oxygen content directly leads to the big difference of properties from the point of technical process. The typical OM micrographs of 1# and 2# are shown in Fig. 2(c) and Fig. 2(d) respectively. The images show that crystalline phase can be found in both samples because the cooling rate is below the critical cooling rate. However the size, fraction and distribution of crystalline phase are completely different. Crystalline phase of 1# is clearly much more and coarser than that of 2#. Only a few of little tiny crystalline phase exists in 2#. The crystalline phase coexisting with amorphous metal can also be reflected from the XRD spectra. As shown in Fig. 2(b), both patterns consist of a broad hump and several sharp diffraction peaks. The crystalline phase is mainly the cubic zirconium solid solution, ZrNb or ZrTiNb. All the diffraction peaks are shifted to the left due to the doping effect. The extent of crystallization of 1# is obviously heavier than that of 2#. Moreover, compared with 2#, there exists a new diffraction peak in the XRD spectrum of 1#.
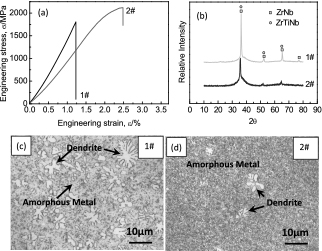
The mechanical properties and microstructure of 1# and 2# samples (a) three-point bending stress-strain curve; (b) the XRD spectra; (c) OM micrographs of sample 1#; (d) OM micrographs of sample 2#.
Obviously, the microstructure of crystalline phase is the key factor to influence the mechanical properties. Too much more and coarser dendrite is exactly the reason that leads to the large difference of plasticity between the two samples. As mentioned above, there is a big difference between 1# and 2# in oxygen content. The high oxygen content increases the tendency of crystallization in BMGs32). The effect of dendrite on the plasticity of amorphous metal was also mentioned in some documents and materials22,33–38). Both dimension and fraction of dendrite play a big role. Therefore the microstructure and morphology of dendrite can be controlled by adjusting the oxygen content so as to improve the mechanical properties of BMGs. Annealing39–41) and cooling rate can also be applied to change the micrographs of dendrite. Compared to adjusting the oxygen content, it is more difficult to adjust dendrite precisely by controlling the process parameters of annealing because the time window around the glass transition temperature is very narrow.
3.2 The effect of cooling rate on the mechanical properties of BMGsFigure 3 shows typical three-point bending stress-strain curve of samples with different thickness and some samples with different plasticity after three-point bending test31). The results indicate that the plasticity of BMGs with the same composition is very scattering with the change of sample thickness. Not only is the strength of sample with 0.8 mm thickness is lowest but also its plasticity is almost zero. The plasticity of sample with 1.4 mm thickness is indeed much better than that of the others. Actually its work-softening behavior is a kind of false appearance caused by the change of gauge length during three-point bending test. In fact, the thickness of the samples essentially affects the cooling rate. The thinner the sample is, the faster the cooling rate is. Therefore the mechanical properties of BMGs are influenced by more the cooling rate than the thickness. And the effect of cooling rate on the plasticity of amorphous alloys is greater than that of oxygen content according to the comparison of Fig. 2(a) and Fig. 3(a).
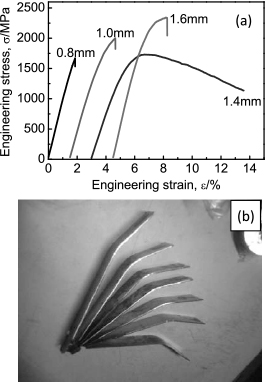
The mechanical properties of samples prepared by high vacuum die-casting (a) typical three-point bending stress-strain curve of samples with different thickness (b) some plastic samples after three-point bending test.
How does the cooling rate influence the microstructure, and then affects the properties, especially the plasticity of amorphous alloys. Figure 4(a) shows the typical XRD patterns of samples with different thickness. That all patterns are broad humps without sharp diffraction peaks indicates the glassy nature of these samples. Therefore, the cooling rate is above the critical cooling rate and the reason leading to the different plasticity of these samples is obviously not same with that of sample 2#. Figure 4(b) shows HRTEM images and the corresponding selected-area electron diffraction (SAED) patterns. The HRTEM images exhibit a homogeneous contrast fluctuation with typical maze-like patterns and the corresponding SAED patterns are perfect halo rings without sharp diffraction spots. Accordingly, samples with different thickness are confirmed the amorphous nature which is in good agreement with the results of XRD. Up until this point we have not detected the microstructural difference, such as phase separation or heterogeneity25,26,42,43), between samples with different thickness. However the plasticity of BMGs can be indeed improved by adjusting the cooling rate. And moreover the cooling rate is not as bigger as better, only the appropriate cooling rate can be effective and efficient to make the brittle metallic glasses obtain high plasticity.
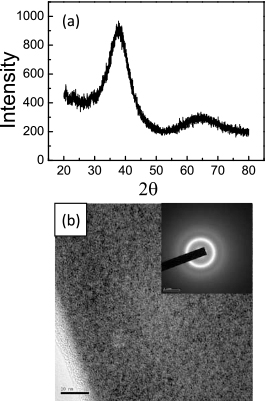
The microstructure characterization of samples prepared by high vacuum die-casting (a) the typical XRD spectrum and (b) HRTEM image inset with the typical corresponding SAED patterns of the samples with different thickness.
How to improve the plasticity of Zr54.6Ti13.8Cu9.3Ni5.8Be13.5Nb3 BMGs through technological means was discussed in this paper. The oxygen content and cooling rate can be adjusted by changing the base pressure of vacuum chamber and thickness of samples respectively. Some conclusions can be drawn as follows according to the experimental results.
(1) The oxygen content can effectively influenced the dimension and fraction of dendrite which has a remarkable impact on the performance of Zr54.6Ti13.8Cu9.3Ni5.8Be13.5Nb3 BMGs.
(2) The plasticity of Zr54.6Ti13.8Cu9.3Ni5.8Be13.5Nb3 BMGs can be improved indeed by adjusting the cooling rate even above the critical cooling rate although we have not detected the microstructural reason. And the plasticity of metallic glass is not monotone changing with cooling rate.
In conclusion, it is technically practical and feasible to adjust oxygen content and cooling rate during preparation of BMGs in order to improve its mechanical properties including plasticity.
The authors would like to thank financial support by Special Research Program of Shaanxi Provincial Department of Education (16JK1382, 16JS044), Transformation of Major Scientific and Technological Achievement of Shaanxi Province (2013KTCG01-04), the National Natural Science Foundation of China (51401155) and Schoolmaster Fund of Xi'an Technological University (XAGDXJJ15007).