2017 Volume 58 Issue 4 Pages 619-622
2017 Volume 58 Issue 4 Pages 619-622
A chromium-based duplex alloy is proposed for a novel class of alloys for hot and harsh environments. The alloy is defined as a duplex microstructure comprised of a hard and high-strength chromium-based BCC (α) phase and a ductile FCC (γ) phase to overcome the brittleness of the conventional α-Cr alloy. The candidate composition of the alloy was selected from a Cr-Fe-Ni ternary system, and the mechanical properties were evaluated. The abrasion and corrosion properties of a surface-remelted chromium-based duplex alloy were compared with those of a Co-based cladding alloy (Bishilite® No. 6), and the results suggested its potential use as a wear and corrosion resistant cladding.
Cladding is used for the surface treatment of components that require wear or corrosion resistant properties against solid/solid friction pairs or a solid/liquid interface. Cobalt (Co)-based claddings are used as bearing metals in hydraulic pumps designed for fluids containing abrasive dirt and corrosive chemicals1–4). However, their protection properties are not enough in a highly-corrosive environment such as piping in oil or gas fields.
In general, the addition of chromium to alloys could improve corrosion resistance5–8). Chromium (Cr)-based BCC (α) alloys were suggested as corrosion-resistant materials9). Passive oxides of chromium, the hard and high-strength α phase matrix, and the reasonable resource price of chromium compared with corrosion resistant cobalt or nickel should be preferred as corrosion resistant claddings with the excellent wear properties in corrosive environments. However, the ductility of the α-Cr alloys is not enough to prevent cracks in the hot forming or cladding process. Low ductility of the α-Cr alloys is recognized to be caused by impurities such as nitrogen because the ductility depends on the post-processing, which includes annealing and a following cooling process. High-purity α-Cr alloys with less interstitial solute atoms are studied to improve the ductility10–12). Alloys with nitrogen less than 0.01 mass% exhibited an improvement in room temperature ductility, and α-Cr alloys with the lowest impurity (0.0015 mass% carbon, 0.0006 mass% nitrogen, and 0.0003 mass% oxygen) showed 20–30% room-temperature elongation. The addition of cerium, titanium, yttrium, and zirconium as impurity scavengers can enhance the ductility13). The mechanism of the brittleness of α-Cr alloys caused by nitrogen was considered as the dislocation pinning of interstitial nitrogen atoms and fine precipitates of nitrides preferentially formed adjacent to grain boundaries10).
In this paper, a Cr-based duplex alloy is proposed for a novel class of alloys for hot and harsh environments. Chromium forms the FCC (γ) phase with the addition of nickel and iron. The γ-phase alloys are generally more ductile than the α-phase alloys. A thermodynamic calculation (Fig. 1, Thermo-Calc2016a, Thermo-Calc Software, with TCFE6 database) predicted the stable α-γ duplex range at 1100℃ for Cr-Fe alloys with the addition of 15 mass% Ni or more. The formation of the intermetallic σ phase around 700–800℃, which causes the embrittlement of the duplex stainless steel, is suppressed in the composition range with less than 30 mass% Fe. We focused on this α-γ duplex range with no σ phase precipitation to obtain Cr-based alloys with benefits originating from the high-strength α-phase and the ductile γ-phase. We selected several compositions for α-Cr and α-γ duplex Cr-based alloys with several α/γ phase ratios to deploy a composition for wear and corrosion resistant cladding.
Fe-Cr-Ni ternary diagram at 1100℃ calculated by Thermo-calc.
Four ingots of Cr-based alloys (Table 1, Fig. 1) were obtained via arc melting (RQM–10T–AW, MAKABE Technical Research Co.). All ingots were cylindrical (ϕ 10 mm–80 mm in length) and weighed 50 g. The raw materials were supplied as chunks (Koujundo Kagaku Co.) and were melted three times to confirm the homogeneity of the ingots. The ingots were heat treated at 1100℃ for one hour in a furnace followed by water quenching to obtain alloys with an equilibrium state at 1100℃, as predicted by thermodynamic calculation (Thermo-Calc 2016a, Thermo-Calc Software, with TCFE6 database). Additional ingots of Cr-based duplex alloys were obtained with induction melting and casting in a water-cooled copper mould (MU-αIV, SK Medical Electronics Co., Ltd., Inc.) followed by surface remelting with a tungsten inert-gas welder (Fronius MagicWave 4000 MV) to obtain the simulated clad metals. The mass of the ingot was set to 80 g for the wear test specimens and to 200 g for the corrosion test specimens. All ingots were characterized by optical microscope (Olympus GX71 and KEYENCE VK-9500) followed by etching with a Murakami reagent (potassium ferricyanide (10 g) and potassium hydroxide (10 g) in pure water (100 g)). The phase ratio was obtained with X-ray diffraction (XRD, Rigaku RINT-2500PC) followed by peak analysis using ICDD PDF-2 database. The average of α phase fraction was calculated from 6 combinations of α phase peaks ((200) and (220)) and γ phase peaks((200), (220) and (311)) (Table 1). Composition of precipitates were analyzed by SEM-EDX (HITACHI, S-3600N). The Vickers hardness test (MMT-X7, Matsuzawa Co. Ltd., 298 K, 2 kgf–15 sec.) and a tensile test (Autograph AG-5000C, Shimadzu, sample geometry; ϕ 2 mm–10 mm in length, 298 K, 1.6 × 10-4/sec) were conducted for mechanical testing. An abrasive wear test for cylinder specimens (ϕ 8 mm–20 mm in length) was conducted to investigate the wear against rotating SiC emery paper (#240) with a load of 39.2 N and a 1.8 m sliding length. The abrasive wear tests were conducted 3 times and specific wear rates were calculated from the changes in the length of samples. Corrosion tests in boiling sulfuric acid (5%) for plate specimens (5 mm × 40 mm × 1.5 mm, grinded by SiC emery paper (#600)) were performed twice for 6 hours. Abrasive wear tests and corrosion tests were conducted for the surface-remelted specimens to evaluate their properties as clad metals compared with Co-based cladding (Bishilite® No. 6 (Table 3), Hitachi Metals Ltd.).
| Sample No. | Cr* | Ni* | Fe* | Mn* | Ti* | Si* | Al* | α-phase fraction** |
|---|---|---|---|---|---|---|---|---|
| D1 | Bal. | 37 | 20 | - | - | - | - | 50.1 ± 19.6 |
| D2 | Bal. | 30 | 20 | - | - | - | - | 62.0 ± 22.2 |
| D3 | Bal. | 25 | 20 | - | - | - | - | 74.1 ± 20.8 |
| A1 | Bal. | 12 | 20 | - | - | - | - | 100 |
| D1R | Bal. | 37 | 20 | 1.5 | 0.5 | 0.5 | 0.02 | 17.0 ± 12.6 |
| D2R | Bal. | 30 | 20 | 1.5 | 0.5 | 0.5 | 0.02 | 77.1 ± 12.5 |
| D3R | Bal. | 25 | 20 | 1.5 | 0.5 | 0.5 | 0.02 | 86.8 ± 5.4 |
* Raw materials-based (mass%), ** Obtained by XRD (vol.%)
The microstructures of the ingots after 1100℃ heat treatment and quenching are shown in Fig. 2. Cracks were found in α-Cr alloy (A1), but all chromium duplex alloys (D1–D3) were successfully obtained without cracks. Bright and dark regions in Fig. 2 corresponded to γ-phase and α-phase regions, respectively. X-ray diffraction analyses allowed identifying both of α and γ phases. Dendrite structures of the primary α-phase and the inter-dendrite γ-phase were found in samples D1 and D2. In D3, the γ-phase was also found as the plate-like precipitates in the primary α-phase. The plate-like precipitates of the γ-phase in the primary α-phase were also identified in D2. Plate-like precipitates of the γ-phase were formed in the α-phase matrix after the solidification of the primary α-phase. The alloy A1 exhibited the dendrite-like contrast that attributed to the elemental segregation. Precipitates such as σ-phase in duplex stainless steel15) and nitrides in Cr based alloys were reported13,16), however, they were not identified in samples D1, D2 and D3 as were checked with the etching with Murakami reagent (σ-phase) and with the SEM-EDX analysis around grain boundaries (nitride).

Microscope images of Cr-based duplex alloys, (a) D1, (b) D2, (c) D3, (d)A1, (e) High magnification D2 and (f) Associated X-ray diffraction diagram of D2.
Breaking elongation, tensile strength and the Vickers hardness of the D1, D2, and D3 specimens after heat treatment (1100℃) and quenching are summarized in Fig. 3. The tensile test specimen for the α-phase alloy (A1) was not successfully obtained because of the cracks in the ingots. Elongation was improved for specimens with fewer α-phase fractions; the D1 ingot exhibited the largest elongation (5.0% at 35.7% α-phase fraction). Considering the crack susceptibility of the α-phase A1 specimen, the introduction of the γ-phase should improve the ductility of Cr-based alloys. The tensile strength and the Vickers hardness exhibited the opposite dependence on the α-phase fraction; the introduction of the γ-phase decreased the tensile strength and the hardness. The abrasive wear resistance of the α-phase A1 was expected to be the best because the rate of abrasive wear is known to be inversely proportional to the Vickers hardness in the same material system17). However, the crack susceptibility of the α-phase A1 should lead to cracks in cladding layers. Therefore, Cr-based duplex alloys such as D2 or D3 were considered as promising cladding metals in this study considering the crack susceptibility during the cladding process.

Mechanical properties of Cr-based alloys; (a) Breaking elongation, (b) Tensile strength and (c) Vickers hardness.
The TIG remelted samples of the Cr-based duplex alloy (D1R, D2R, and D3R) were obtained as simulated clad metals to compare the properties with Bishilite® No. 6 claddings (Fig. 4). The α-γ duplex structures were obtained in D1R, D2R and D3R, and the constituting phases were the same as the cast samples. However, the plate-like γ-phase precipitates were not observed in the primary α-phase. The higher cooling rate during solidification in the remelt process than in the arc-melt process and the absence of a heat treatment at 1100℃ were supposed to suppress the precipitation of the γ-phase in the primary α-phase. Some oxide precipitates were observed in TIG-remelted alloys(Fig. 4(d)(f)).

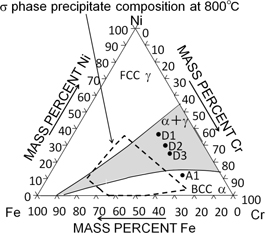
Microstructures of TIG-remelted Cr-based duplex alloys, (a) D1R, (b) D2R (c) D3R (d) SEM image of precipitate in D2R, (e) Associated X-ray diffraction diagram of D2R and (f) Composition of precipitate in (d) obtained by SEM-EDX.
Properties as clad metals, the hardness, the wear rate in an abrasive wear test, and the corrosion rate in boiling sulfuric acid were obtained for D1R, D2R, and D3R specimens to compare with the properties of Co-based Bishilite® No. 6 cladding (Table 2). D1R and D2R exhibited a higher Vickers hardness than that of Bishilite® No. 6. All of the Cr-based duplex alloys showed a lower abrasive wear rate than that of Bishilite® No. 6. D3R exhibited a superior abrasion resistance than that of Bishilite® No. 6 although the Vickers hardness was lower. Cross sections of D2R and Bishilite® No. 6 were observed to consider the mechanism of the abrasive wear (Fig. 5). Bright and dark regions in Bishilite® No. 6 corresponded to cobalt solid solution and carbide regions. Carbides were smaller than the hard α-phase in D2R. The abrasive wear rate of Bishilite® No. 6 was thought to be higher because carbide was dug up by SiC grains on the emery paper. The abrasive wear behavior in the Cr-based duplex alloy will be carefully investigated in future work.
| D1R | D2R | D3R | Bishilite 6 | |
|---|---|---|---|---|
| Vickers hardness (Hv) | 256 | 593 | 777 | 465 |
| Specific wear rate, | 3.0 ± 0.2 | 2.7 ± 0.4 | 2.1 ± 0.2 | 3.4 ± 0.3 |
| Swr/10−11m3·(N·m)−1 | ||||
| Corrosion rate, Cr/g·m−2·h−1 | 1.6 ± 1.6 | 0.15 ± 0.08 | 0.30 ± 0.09 | 151 ± 0.67 |
| Co | Ni | Cr | W | Fe | C | |
|---|---|---|---|---|---|---|
| Bishilite 6 | Bal. | ≦3 | 28 | 4 | ≦3 | 1 |

Cross section BSE images, (a) D2R, (b) High magnification D2R, (c) Bishilite® No. 6, (d) High magnification Bishilite® No. 6.
All of the remelted Cr-based duplex alloys in this study exhibited great improvement in the corrosion rate in boiling sulfuric acid, which was 0.10–1.1% of the rate of Bishilite® No. 6 (Table 2). It arose from the passive films formed on chromium-based alloys. The specimens after the corrosion test are characterized (Fig. 6). D2R and D3R specimens clearly kept their silver colored metallic luster and abrasive flaws originating from the sample machining, and no significant changes in appearance were confirmed. The Bishilite® No. 6 cladding reacted with sulfide acid and it was covered with black corrosion products. The passivation films seemed to prevent such corrosion products formation on D2R and D3R. The metallic luster on D1R turned dull, and the abrasive flaws became invisible after the corrosion test; however, no corrosion products were observed on the surface. The selective dissolution of the γ-phase was observed on D1R. The corrosion rate of D1R was higher than D2R and D3R. The corrosion resistance in boiling sulfuric acid for the Cr-rich α-phase was found to be superior to the γ-phase in all Cr-duplex alloys.

Microstructures of specimens after corrosion tests (a) D1R, (b) D2R, (c) D3R and (d)Bishilite® No. 6.
The investigation in this study revealed an improvement in ductility for Cr-based alloys by the introduction of a α-γ duplex microstructure. The improved ductility of the Cr-based duplex alloys suppressed crack formation during the remelting process. The developed Cr-based duplex alloys successfully exhibited an improved abrasive wear resistance and corrosion resistance in boiling sulfuric acid than those of the conventional Co-based claddings (Bishilite® No. 6). Cladding of a test piece with the chromium duplex alloy is currently under development to demonstrate its properties as cladding metals. We will continue the development of this alloy and reveal its profound capabilities as a new class of materials for extreme environments.
The authors are grateful to Dr. Yasuhisa Aono for his theoretical guidance and thoughtful discussion from the very early stage of this work.