2018 Volume 59 Issue 10 Pages 1659-1664
2018 Volume 59 Issue 10 Pages 1659-1664
High-percentage iron resources in nickel slags were recovered as magnetite via molten oxidation process, and the transfer behavior of Fe element was studied. The elemental distribution in oxidized slag samples, the influence of atmosphere, holding temperature and time on magnetite crystal growth, and Fe element distribution in magnetic materials were also investigated. It was found that magnetite could be produced from fayalite or hortonolite in nickel slags during molten oxidation with CaO as a modifier, air as an oxidizer, accompanying with the enrichment of Fe, Co, Ni and Cu. The select of atmosphere is very important during the precipitation and growth of the magnetite crystals. The magnetite crystals precipitated invisibly or slightly in argon atmosphere, while exhibited dendritic structures with crystallization content of ∼18.5% in air atmosphere. Especially, after blowing air into molten slag for 30 min, magnetite crystals develop well-distributed and complete, resulting in its crystallization content increases up to 33.5%. The Fe content in the matrix of oxidized samples remained approximately constant after holding for 20 min. Mössbauer spectra analysis indicates that the 89.6% of Fe exists in magnetite phases, while only 10.4% of Fe in hedenbergite. It was also found that Ni and Co simultaneously concentrate in the magnetite phase, indicating that Fe, Ni, and Co can be recovered effectively from nickel slag.
Slags produced during large-scale nickel metallurgy are industrial waste, but contain significant iron element (∼40%) and other valuable elements including Ni, Co, and Cu. Nickel slags are generated at a rate of ∼16 tons per ton of nickel production.1,2) These slags stack so large that give rise to environmental pollution and resources waste. In contrast, iron ore containing more than 29.1% iron content is of value for exploring, and accompanies large quantities of associated minerals.3) Therefore, the study of iron resource utilization in nickel slags has much attention to research due to its theoretical research value and economic and environmental benefits.
Fayalite (2FeO·SiO2) is the main iron-rich phase in nickel slags.4,5) This iron resource cannot be used directly owing to its stable structure, resulting from a complex silicate formed by interlinked Si–O atoms.6–8) Recent work has been performed for investigating the transformation of fayalite to magnetite via oxidation process for iron element recycling.9,10) Y. Fan et al.11) studied the phase transformation and the crystallization behavior of the magnetite phase in molten copper slags, showing that a lower oxygen partial pressure was conducive to the magnetite precipitation. H. Cao et al.12) also studied the transformation behavior of fayalite to magnetite in molten copper slags, as well as how magnetite precipitated. It was reported that a higher oxygen flow and oxidative temperature were beneficial to iron element transfer and enrichment in copper slags.
It is well known that the composition and properties of nickel are different from copper slags due to its higher MgO, Ni, Co, and Cu content. Our research group previously reported that crystallization and beneficiation of magnetite for iron recycling from nickel slags by oxidation-magnetic separation.13) However, the Fe, Co and Ni element distribution in oxidized slag samples, the influence of different atmosphere on magnetite crystal precipitation and growth, as well as the distribution of Fe element in magnetic materials were not investigated. Therefore, it is necessary to investigate iron element transfer behavior during molten oxidation and magnetic separation in nickel slags.
The materials, appeared as ash black particles and used for the experiment were taken from water-quenched nickel slags (original nickel slags) in flash furnaces (Jinchuan Group Co., Ltd), and their composition is listed in Table 1. It can be seen that the total iron (TFe) content of the slag is up to 40%, and other valuable elements including Ni, Co, and Cu are also contained. Analytically pure CaO was employed as a modifier to destroy the structure of fayalite. SiO2 saturation in nonferrous slags can be written as follows:14)
| \begin{equation} Q = w_{\textit{SiO}_{2}}/(w_{\textit{MgO}} + w_{\textit{FeO}}) \end{equation} | (1) |

The nickel slags were crushed and screened into particles smaller than 200 µm in diameter. 100 g of nickel slag and 16.67 g of CaO, i.e. a ternary basicity ($(w_{\textit{MgO}} + w_{\textit{CaO}})/w_{\textit{SiO}_{2}}$) of 0.9, were mixed together and pressed into tablets. The tablets were then placed into a corundum crucible in a high-temperature furnace and heated to 1450°C at a rate of 5°C/min. The slags were melted and oxidized with air blowing into molten slag at flow rate of 200 mL/min. After holding for 30 min, the oxidized slags were cooled to 1000°C at a cooling rate of 5°C/min, and then cooled naturally down to room temperature in the furnace. Finally, the oxidized slags were ground into small (<74 µm diameter) particles and underwent magnetic separation. The magnetic materials were finally obtained via magnetic separation in a magnetic tube with a magnetic field intensity of 0.3 T. A schematic diagram of the experiment is shown in Fig. 1.

Schematic diagram of experiment.
A phase analysis of the samples was conducted using X-ray diffraction (XRD) while the characterization of microstructure was performed using electron probe microanalysis (EPMA). A Zeta Absorption Fluorescence (ZAF) method was adopted for quantitative analysis, and GH915, GH4169 and GH4049 were used as standard samples. By using image analysis software (Image Pro Plus), the sizes of particles in the magnetite phases and the degree of crystallization were measured. The element content of the sample was analyzed by X-ray fluorescence (XRF), and the iron content in different phases was analyzed by Mössbauer spectroscopy.
Figure 2 shows backscattered electron (BSE) images of the original nickel slags. Three phases are shown as a gray lath, a matrix, and a dot or rod shape, denoted “a”, “b”, and “c”, respectively. It can be found that iron element is mainly concentrated in gray strips from surface scanning analysis as shown in Fig. 3. The matrix, consisting of amorphous silicate glass phases, can be found in the area of lath-shaped fayalite intervals. The dots or rods are attributed to the presence of the sulfide phases of copper, nickel, and iron. Figure 4 shows the XRD pattern of the original slags, indicating that fayalite and hortonolite are main phases, which is in good agreement with above calculated results.

BSE image of original slags.
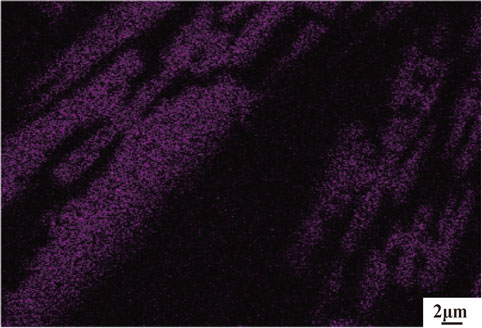
Fe surface scans image of original slags.

XRD pattern of original nickel slags.
Generally, oxides can exist as two different states in molten slag: one is free simple oxide molecules, the other is complex compounds.15) Fayalite is a type of reticular complex compound, and the addition of alkaline oxide can destroy its reticular structure.9) Therefore, we choose CaO as a modifier to destroy fayalite structure. In the molten slag, CaO can release free oxygen ions (O2−) because O2− easily escaped from the bound cationic Ca2+ at high temperature. The oxygen ions can destroy the reticular structure of 2FeO·SiO2 and release FeO, as well as bring the change of slag fluidity. The reticular structure fayalite will thus transform into simple silicon oxide anions such as SiO42−. This indicates that the viscosity activation energy (Eη) decreases because the transfer of SiO42− is more easily than that of fayalite, according to the Arrhenius viscosity, as shown in eq. (2).
| \begin{equation} \eta = \eta_{0}\cdot\exp[E_{\eta}/(\mathrm{R}T)] \end{equation} | (2) |
The reduced Eη was conducive to the decreasing of the viscosity, thus improves the slag fluidity. In addition, the Ca2+ dissociated from the CaO combine with SiO42− to form 2CaO·SiO2, and the O2− combined with Fe2+ to form FeO as shown in reaction (3). This means that CaO can replace FeO in 2FeO·SiO2 and cause to release free FeO.
| \begin{equation} (\text{2FeO$\cdot$SiO$_{2}$}) + \text{2CaO$_{(s)}$} = \text{2(FeO)} + (\text{2CaO$\cdot$SiO$_{2}$}) \end{equation} | (3) |
| \begin{equation} \text{3(FeO)} + \text{1/2O$_{2(g)}$} = (\text{Fe$_{3}$O$_{4}$}) \end{equation} | (4) |
Figure 5 shows a BSE image of oxidized slags. Many white-grey phases precipitated from the dark grey matrix. The EDS analysis shows that the precipitated phases marked “a” contained primarily Fe, and the dark-matrix “b” region contained Si, Ca, Fe, Mg, and Al, as shown in Table 2. The line-scan patterns of the oxidized slags are shown in Fig. 6. Iron element existed primarily in the white grain, while Si and Ca elements existed in the matrix. The XRD pattern of the oxidized slags was shown in Fig. 7, and indicated that the white-grey phases of the sample could be attributed mainly to the magnetite phases, while the matrix consisted of glassy phases containing various elements such as Si, Ca, Fe, and Mg. In addition, the Ni, Co, and Cu contents of the magnetite phases are higher than those in the matrix, similar to Fe behavior. This may be attributed to isomorphism phenomenon, since Fe, Co, and Ni, belong to the VIII family and Cu belongs to the IB family in the periodic table.18) Co, Ni and Cu replaced Fe in the magnetite phases, causing the standard peak of Fe3O4 to shift toward right in the XRD pattern.19) Thus, it can be concluded that the fayalite in nickel slags transform effectively into magnetite phase after molten oxidation, accompanying simultaneously Fe, Ni, Co, and Cu enrichment.

BSE image of oxidized slags.


Line-scan patterns of different elements in oxidized slags.

XRD pattern of oxidized slags.
Figure 8 shows the BSE images of the magnetite crystals in different atmosphere. According to Delesse’s law, the volume fraction of the magnetite phases in the component can be expressed as the area fraction occupied by the magnetite phases on a cross-section, which is defined as crystallization content.20) Through analytic statistics of 5–7 visual fields that were randomly selected from the sections of the slags specimens by IPP software, the percentage of magnetite coverage was calculated, and the average value was adopted as crystallization content. In argon (Ar) atmosphere, the magnetite crystals precipitated invisibly or slightly, as shown in Fig. 8(a). In air atmosphere, magnetite crystals in the slags developed incompletely and formed dendritic structures, with the crystallization content calculated by statistical software as ∼18.5%, as shown in Fig. 8(b). After introduced air into molten slag via a corundum tube for 30 min at flow rate of 200 mL/min, the magnetite crystals developed completely and formed granular structures with uniform distributions, and crystallization content increased up to 33.5%, as shown in Fig. 8(c). Thus, the select of atmosphere was very important to the precipitating and growing of magnetite crystals. The reason was probably that the high oxygen content can promote the transformation of FeO to Fe3O4, accompanied the occurring of different morphology or structures of the magnetite crystals. It should be mentioned that the presence of air could also play an important role in improving the dynamics of the oxidation reaction due to its stirring to the bath.

BSE images of slags in different atmosphere. (a) Ar atmosphere (b) air atmosphere (c) introduced air.
Oxidized slags were held at 1450°C (1723 K) then cooled down to different temperatures at ∼50°C/min by heating halting and blowing argon into the heating chamber. Samples were taken out after different holding time at 1623 K, 1673 K, and 1723 K. The relationship between iron content in the slag matrix and the holding time at different holding temperatures is shown in Fig. 9. At all holding temperatures, the iron content in the matrix decreases rapidly within 20 min, and remains approximately constant afterward. This means the magnetite precipitation in the oxidized slags has reached equilibrium after holding for 20 min. In addition, the iron content in the matrix decreases slightly for the same holding time with increasing holding temperature. The viscosity of the slags at lower temperature is less than that of at higher temperature, which favors the reaction dynamics and magnetite precipitation, resulting in lower iron content in the matrix.

The curves of iron content in slag matrix and holding time at different temperatures.
After magnetic separation of the oxidized slags, the elemental contents of the magnetic materials were analyzed by XRF, the results of which are shown in Table 3. Due to synergy action of modification and oxidation, the Fe, Ni, and Co contents of the magnetic materials are much higher than that of the non-magnetic materials.

Figure 10 shows the Mössbauer spectra of Fe in the magnetic materials. The 89.6% of Fe exists in magnetite phases, with 50.8% of Fe3+ and 38.8% of Fe2+, while only 10.4% of Fe in hedenbergite, as shown in Table 4. Thus, it is further proved that Fe in the nickel slags transforms effectively from fayalite into magnetite after molten oxidation process, and Ni and Co simultaneously concentrate in the magnetite phase, indicating that Fe, Ni, and Co can be recovered effectively from nickel slag.

Mössbauer spectra of Fe in magnetic materials.

The authors gratefully acknowledge the National Natural Science Foundation of China (No. 51374062, 51574065, 51774073), Science and Technology Major Projects of Gansu Province (No. 145RTSA004), and Youth Science and Technology Foundation of Gansu Province (No. 17JR5RA116), who made this research possible.