2018 Volume 59 Issue 4 Pages 690-693
2018 Volume 59 Issue 4 Pages 690-693
The change in TiO2 solubility in molten CaCl2 at 1573 K with CaO addition was studied to clarify the influence of CaO on TiO2 dissolution. The solubility of TiO2 in molten CaCl2 remarkably enlarged with CaO addition, which suggests that a titanate ion (TixOyZ−) was preferentially formed in it. The cathodic behavior in molten CaCl2 containing various species of calcium titanate at 1373–1573 K was investigated by cyclic voltammetry, and electrodeposition of Ti metal was attempted by potentio-static electrolysis. The reduction behavior was affected by the species of calcium titanate, and the components in the deposit changed consequently. These results indicate that the reduction behavior of Ti was strongly influenced by the species of titanate ion, and that the bath consisting of Ca3Ti2O7 is found suitable.
A new efficient process of Ti production replacing the Kroll process is desired. Many researchers have been studied the innovative smelting processes,1–8) but those processes are still under developing. The authors also proposed the direct electrowinning of liquid Ti from TiO2 considering its advantage in term of productive efficiency. From the results using molten fluoride bath, it was suggested that reduction behaviors of Ti were strongly affected by the state of Ti in the bath; a oxy-titanate (TixOyz−, representing just “titanate”, hereinafter) ion seemed to be preferentially formed, and the reduction behavior of Ti changed with the species of a titanate ion.9) It is generally said that metal oxide hardly dissolves in chloride melt, and some researches on Ti metal production in CaCl2 melt1–7) were proposed with the supposition that TiO2 was insoluble in it. However, the results in fluoride melt mentioned above suggest the formation of a soluble titanate ion by the addition of calcium titanate in CaCl2 melt. From this point of view, it was already shown that CaTiO3 could dissolve in CaCl2 melt above 1373 K, and that Ti metal was detected in the electrodeposit.10)
In this paper, the change in the dissolution of a sintered compact of TiO2 with CaO addition in CaCl2 melt was studied to clarify the dependence of TiO2 dissolution on the O2− existence in CaCl2 melt first. Using three species of calcium titanate, CaTiO3, Ca4Ti3O10 and Ca3Ti2O7, the cathodic behavior in CaCl2 melt containing them was also measured, and the dependence of the cathodic behavior on the species of a titanate ion was discussed.
The same apparatus as our previous study10) was used. For the study on the change in the cathodic behavior by the species of a titanate ion, three calcium titanates: CaTiO3 whose molar ration of CaO to TiO2(RCaO/TiO2) was 1.0, Ca4Ti3O10 (RCaO/TiO2 = 1.325) and Ca3Ti2O7 (RCaO/TiO2 = 1.5), were used, because these compounds are reported as stable in the reported.10,11) These calcium titanates were prepared in our laboratory by sintering a molded mixture of CaO (Kishida Chem., >98%) and TiO2 (Kishida Chem., >99.5%), analyzing by XRD (Rigaku, RINT-2550V) and then grinding to powder.
Calcium chloride (Kishida Chem., >95%) about 40 g with the calcium titanate was put in a Mo crucible, and vacuum dried at 373 K for a day. The mixture with the crucible was set in an air-tight furnace (Motoyama, MS-2821), and melted at 1573 K under a pure Ar flow. The bath temperature was varied between 1373 K–1573 K to study the influence of temperature. For the investigation on the influence of the CaO addition on the TiO2 dissolution, a sintered compact of TiO2 at 1573 K was immersed in CaCl2 bath with/without CaO after its melting.
The cathodic reaction was investigated by cyclic voltammetry. A Mo wire (Nilaco, ϕ1.0 mm) covered with a pure Al2O3 tube was used as a working electrode, and a graphite rod was used as a counter electrode. Another Mo wire was used as a quasi-reference electrode. The potential of the quasi-reference electrode was calibrated with Mo dissolution potential, whose value was about 2.2 V nobler in comparison with the Ca/Ca2+ potential used in our previous study.9,10) Based on the results by cyclic voltammetry, potentio-static electrolysis was carried out. The electrodeposit was washed with ethanol, and analyzed by XRD and SEM-EDX (JEOL, JCM-6000).
Figure 1 shows the change in the weight loss of the TiO2 compact after immersion in CaCl2 melt at 1573 K for 2 hours. On the surface of the compact after the immersion, CaTiO3 was detected. The weight loss in the bath without CaO was very small, while the weight remarkably decreased in the bath with CaO. The larger the added CaO amount was, the larger the weight loss was. However, the weight loss of the compact was much smaller than the expected value from the solubility of calcium titanate mentioned after, which implies the dissolution rate of the TiO2 compact was not high.

Relationship between weight loss of TiO2 sintered compact and CaO concentration.
Current density in a cyclic voltammogram slightly increased with TiO2 powder addition, and was remarkably enlarged by CaO existence as shown in Fig. 2. These results suggest that the existence of CaO in the bath remarkably accelerated the TiO2 dissolution.
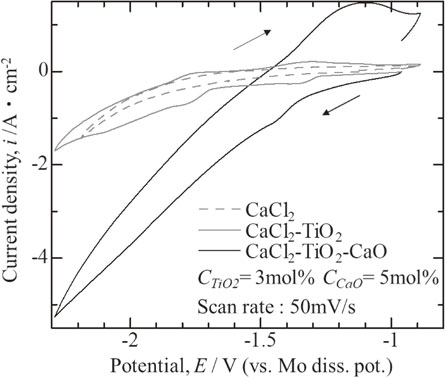
Change in cyclic voltammogram in bath with TiO2 and CaO addition.
There is a possibility that TiO2 dissolved as four species: a TiO2 molecule, a Ti cation (Ti4+/TiO2+), a Ti oxy-chloride complex ion (TiO2Clnn−) and a titanate ion. If TiO2 dissolved as a TiO2 molecule or a Ti cation, the solubility was not affected by the CaO addition directly. The existence of an O2− ion should inhibit the dissolution as a Ti cation. Since a Cl− plentifully exists in the bath in spite of the CaO addition, the dissolution as an oxy-chloride ion hardly explains the influence of the CaO addition. Since cathodic current was slightly increased by the immersion of TiO2 compact in the bath without CaO, TiO2 can dissolve as a TiO2 molecule, a Ti cation or a oxy-chloride ion to some extent. However, the remarkable influence of CaO addition on the TiO2 dissolution in Fig. 1 and Fig. 2 strongly suggest that TiO2 mainly dissolved as a titanate ion.
Various ions with different Ti:O ratios may exist in the bath though the real formula of the dissolved titanate ion has not been clarified yet. The derived ions from CaTiO3, Ca4Ti3O10 and Ca3Ti2O7 were supposed in this paper.
3.2 Change in cyclic voltammogram with calcium titanate additionThe shape of cyclic voltammogram was varied by the species of added calcium titanate as shown in Fig. 3, though clear current peaks were hardly observed probably due to convection in the melt under the high temperature condition, 1573 K. Three current peak/humps were seen at −1.2 V, −1.7 V and −2.0 V in the melt with CaTiO3. The current peak/humps at −1.2 V seemed to shift to −1.0 V in the melt with Ca4Ti3O10 and Ca3Ti2O7, and its shape became clear. The peak/hump at −1.7 V slightly moved to −1.6 V, and that at −2.0 V disappeared in the melt with Ca4Ti3O10 and Ca3Ti2O7. These results agree with those in the fluoride melt,9) and suggest that the cathodic behavior of Ti was changed by the species of a titanate ion.

Change in cyclic voltammogram with species of calcium titanate.
Figure 4 shows the change in cyclic voltammograms with Ca3Ti2O7 concentration calculated from its added amount. The current density overall increased with the concentration, but the current in the 10 mol% bath was rather lower than that in the 7 mol% bath. The cathodic current peak at −0.95–−1.1 V in Fig. 4 was thought to correspond to the same reaction on Ti reduction. Figure 5 shows the relationship between the peak current density at −0.95–−1.1 V and the added Ca3Ti2O7 concentration. The peak current at 1573 K linearly increased with the added Ca3Ti2O7 concentration below 7 mol%, and became rather small in the bath of 10 mol%-Ca3Ti2O7. This result can be explained by considering the solubility of Ca3Ti2O7 in CaCl2 melt is about 7 mol% at 1573 K. The solubility at 1373 K and 1473 K was also estimated as 5 mol% and 7 mol%, respectively. The plotting of cathodic current at −1.8 V against the added amount of Ca3Ti2O7 as shown Fig. 6 gave almost the same solubility. These values were consistent with those of CaTiO3 measured at −1.8 V, though a mole of Ca3Ti2O7 contains Ti twice in comparison with that of CaTiO3.

Change in cyclic voltammogram with Ca3Ti2O7 concentration. (T = 1573 K, scan rate: 50 mV/s).

Relationship between peak current density around −1.0 V and Ca3Ti2O7 concentration. (scan rate: 50 mV/s).

Relationship between current density at −1.8 V and Ca3Ti2O7 concentration. (scan rate: 50 mV/s).
The results above indicate that various species of calcium titanate dissolved in molten CaCl2 above 1373 K, and their solubilities depended on their species as well as temperature.
3.3 Influence of calcium titanate on Ti electrodepositionIt was reported that Ti metal was obtained potentio-static electrolysis at −2.2 V in the bath with CaTiO3, but undersiable Ca metal seemed to deposit simultaneously under the condition; Ca metal is said to form metal fog easily, and Ca metal fog may cause Ti metal dispersion which is undesirable to our ultimate purpose,10) liquid Ti metal deposition. To prevent Ca metal deposition, potentio-static electrolysis at −1.4, −1.5 and −1.6 V was carried out in the bath with CaTiO3, but Ti metal was not detected in the deposit. From these results, the chemical reduction to Ti metal by electrodeposited Ca metal in the electrolysis at −2.2 V cannot be denied, and direct electrochemical reduction to Ti metal may be hardly achieved in this bath.
Titanium metal was obtained by potentio-static electrolysis at −1.7 V and −1.8 V in the melt with 3–7 mol% Ca4Ti3O10. Ca was not detected in the deposit, but some types of lower-valent Ti oxides were contained together. Figure 7 shows the XRD pattern of the electrodeposit obtained by the electrolysis at −1.9 V in the melt with Ca3Ti2O7. The potential region where Ti metal was obtained without Ca co-deposition was −1.9–−2.0 V in this bath, but lower-valent Ti oxides were still detected in the deposit. The electrolysis condition to deposit pure Ti metal should be clarified.

XRD pattern of electrodeposit obtained in bath with Ca3Ti2O7. (CCa3Ti2O7 = 5 mol%, E = −1.9 V, T = 1573 K, t = 7200 s). (Cu-Kα, 40 kV, 300 mA).
It was shown that the species of calcium titanate strongly affected Ti metal deposition, and that Ti metal was obtained in CaCl2 melt of RCaO/TiO2 above 1.325. It was reported that Ti metal deposition strongly depended on the RCaO/TiO2 in fluoride melt,9) and that Ti metal was deposited efficiently at the limited RCaO/TiO2 around 1.5.8) Taking the results by this study and those in fluoride melt into condideration, Ca3Ti2O7 is thought suitable as the solute for Ti metal deposition in CaCl2 melt at present.
The existence of CaO in molten CaCl2 remarkably accelerated the TiO2 dissolution in it, which suggests that a titanate ion was preferentially formed in the bath. The solubility of Ca3Ti2O7 was estimated from the cyclic voltammograms, and its value seemed almost the same outwardly with the reported values on CaTiO3. The reduction behavior changed with the species of added calcium titanate, and the electrodeposition of Ti metal depended consequently. Ca3Ti2O7 seemed suitable for Ti metal deposition in CaCl2, which agrees with the results reported in CaF2-CaO-TiO2 melt.
This study was supported by the New Energy and Industrial Technology Development Organization (NEDO) under the “Innovative Structure Materials Project (Future Pioneering Projects)”, and by Kansai University Grant-in-Aid for progress of research in graduate course, 2011.