2018 Volume 59 Issue 4 Pages 568-574
2018 Volume 59 Issue 4 Pages 568-574
A molecular dynamics (MD) simulation of the solid-state bonding between single crystals of bcc iron and fcc nickel, i.e., dissimilar components, was conducted by hot-pressing with various initial compressive strains ranging from 14 to 20% and subsequent uncompressed isothermal holding at 873 K. Then, the intrinsic strength of the interfaces with various isothermal holding times was evaluated by uniaxial tensile technique. It was found that the interface separation follows the traction-separation law and that always take place either at the interface or close to the interface. The intrinsic strength of the interface is very low under an as-compressed condition and tends to rapidly increase in the early stage of isothermal holding. In addition, lower intrinsic strength was observed in a specimen with higher initial compressive strain. The significant increase in the intrinsic strength is attributed to the short-range atomic rearrangement of a layer of disordered atoms at the interface, driven by energy stored from the compressive deformation.
Molecular dynamics (MD) simulation has been widely utilized to investigate the atomic behavior of interfaces, surfaces, and grain boundaries1), owing to the limitations of experimental observation at the atomic scale. For example, the energy of symmetrically tilted grain boundaries of single-crystal body-centered-cubic (bcc) and face-centered-cubic (fcc) crystal structures of iron has been calculated2), showing that no premelting phenomenon occurs at temperatures near the melting point in some special symmetrically tilted boundaries. Also, several mechanical properties and deformation behaviors of metals at the atomic scale have been evaluated by atomistic simulations to investigate on the characteristics of plastic and elastic deformation near an interface under tensile and compressive loads3,4).
From our previous study of the solid-state bonding of interstitial-free (IF) steel at relatively low temperatures5), the evolution of the interfacial strength can be divided into two stages: a rapid first stage and a more gradual second stage. Regarding to the rapid increase in interfacial strength in the first stage, the dominant mechanism was found to occur at contact area deformed by compression without any long-range diffusion. In addition, an experiment to examine the solid-state bonding between IF steel and pure nickel under varied compressive strain at a relatively low temperature revealed that the decrease in the yield stress of the microstructure adjacent to the interface played an important role in the evolution of the strength per contact area in the first stage by promoting plastic energy dissipation during the interface fracture6). Referring to the interface fracture theory based on the embedded process zone (EPZ) model7,8), not only does the yield stress of the microstructure adjacent to the interface affect the strength per contact area but also the increase in the intrinsic strength play an essential role in the evolution of the strength per contact area in the first stage.
To observe the change in the atomic arrangement at the interface, an atomistic simulation of the solid-state bonding between single crystals of bcc iron atoms at a low temperature was investigated by MD simulation using the Finnis-Sinclair (FS) potential energy5). Short-range movement of the atoms adjacent to the interface was observed, which appears to induce continuity between the lattices of the bonding components as isothermal holding time increased. This leads to a significant decrease in the potential energies of the simulated specimens and greater stability of the atomic arrangement near the interface. Nevertheless, the evolution of the intrinsic strength of the interface in very early stage of the bonding is still unclear.
The aim of this study is to clarify the relationship between the progress of the atomic arrangement near the interface and the evolution of intrinsic strength of the interface in the very early stage of solid-state bonding between dissimilar metal components at relatively low temperatures, and to investigate the effect of the initial compressive strain on the atomic arrangement, as well as the intrinsic strength of the interface, by numerical analysis. In addition, the mechanical properties of the bonding interface are investigated in more detail by performing uniaxial tensile loading as well as potential energy analysis during isothermal holding.
A classical MD simulation was performed to investigate the solid-state bonding process between single-crystal bcc iron and fcc nickel. The embedded-atom method (EAM) potential for an alloy system developed by Zhou et al.9) was employed. The potentials are well-defined for basic material properties, including lattice constants, elastic constants, bulk moduli, vacancy formation energies, sublimation energies, and dislocation line energies. The total energy of this EAM potential, E, is expressed as follows:
| \[ E = \sum_{n} E_{i}, \] | (1) |
| \[ E_{i} = \frac{1}{2} \sum_{i \ne j} \emptyset_{ij} (r_{ij}) + \sum_{i} F_{i} (\rho_{i}), \] | (2) |
| \[ \emptyset (r) = \frac{A \exp[- \alpha (r/r_{e} - 1)]}{1 + (r/r_{e} - K)^{20}} - \frac{B\ \exp[- \beta (r/r_{e} - 1)]}{1 + (r/r_{e} - K)^{20}}, \] | (3) |
| \[ F(\rho) = \left\{ \begin{array}{l@{}} \displaystyle \sum\limits_{i=0}^{3} F_{ni} \left( \frac{\rho}{\rho_n} - 1 \right)^{i}, \quad \rho < \rho_{n}\\ \displaystyle\sum\limits_{i=0}^{3} F_{i} \left( \frac{\rho}{\rho_{e}} - 1 \right)^{i}, \quad \rho_{n} < \rho < \rho_{e} \end{array} \right., \] | (4) |
| \[ \rho_{i} = \sum_{j,j \ne i} f_{j} (r_{ij}), \] | (5) |
| \[ f(r) = \frac{f_{e} \exp [- \beta (r/r_{e} - 1)]}{1 + (r/r_{e} - \lambda)^{20}}, \] | (6) |
| \[ \emptyset^{ab} (r) = \frac{1}{2} \left[ \frac{f^{b}(r)}{f^{a}(r)} \emptyset^{aa} (r) + \frac{f^{a}(r)}{f^{b}(r)} \emptyset^{bb} (r) \right], \] | (7) |
In the simulation, the leapfrog method was used with a time step of 5.0 fs. Assuming a periodic boundary, the number of atoms, the simulation volume, and the temperature were set to be constant in each time step. In this study, the lattice parameters of bcc iron and fcc nickel were assumed to be 2.8665 Å and 3.52 Å, respectively.
2.2 Specimens and thermomechanical processEach simulated bonding specimen consisted of two parts: a lower bcc iron lattice, whose [010] and [001] were parallel to the y and z axes, and an upper fcc nickel lattice, whose [010] and [001] were tilted by 25° relative to the y and z axes, respectively. The dimensions of the simulated specimens, which had a thickness of 35.2 Å, are illustrated in Fig. 1. The total number of atoms in the system was approximately 160,000. The interface energy of the interface without deformation was investigated and no significant cusp in the interface energy was found; thus this specimen potentially represents a typical high-angle interface between bcc iron and fcc nickel.

Simulated bonding specimen with bcc iron lattice, whose [010] and [001] directions are parallel to the y axis and z axis, and the fcc nickel lattice, whose [010] and [001] directions are tilted by 25° relatively to the y axis and z axis, respectively.
The thermal history of the thermo-mechanical process used in the simulation is illustrated in Fig. 2. Lattices of bcc iron and fcc nickel were prepared at 0 K then heated to 873 K at a rate of 8.0 × 1013 Ks−1. Subsequently, the specimens were isothermally held for 100 ps for the stabilization of temperature before their bonding. Compression with a compressive strain of 14–20% was then temporarily applied at 873 K with a strain rate of 5.0 × 106 s−1 along the z axis, which was followed by isothermal holding for up to 10000 ps without pressure. Then, the intrinsic strength of the interface of the bonding specimen was calculated for various isothermal holding times by uniaxial tensile loading with a constant strain rate of 10−3 ps−1 at a temperature of 10 K until fracture occurred.

thermo-mechanical process used in the MD simulation.
Figure 3 shows the relationship between the separation and the tensile stress along the z axis of the specimen with an initial compressive strain of 18%, which suggests that the relationship tends to follow the traction-separation law of interface separation10). The stress steadily increases with increasing separation from the beginning of uniaxial tensile loading and reaches a maximum value, defined as the peak stress. Subsequently, crack propagation begins and the contact area starts to decrease until complete separation. It can be seen that deformation during the crack propagation is more significant in the specimen with a long isothermal holding time than in the specimen under an as-compressed condition. Note that the calculated stress in this study does not only refer to the stress in z axis of atoms close to the interface, but to the stress in z axis of uppermost layers of atoms of the upper part and lowermost layers of atoms of the lower part.

Stress obtained by uniaxial tensile loading perpendicular to the interface of specimens with initial compressive strain of 18% under as-compressed condition and isothermal holding of 200 and 5000 ps in relationship with separation.
The relationship between the isothermal holding time and the peak stress of specimens with initial compressive strains of 14–20% is shown in Fig. 4. After the compression, the peak stress of the specimen under an as-compressed condition is low and tends to continuously increase with isothermal holding. From Fig. 4, linear relation can be appreciated in the evolution of the peak stress with the exponential increase in isothermal holding time in the early stage, meaning that the peak stress rapidly increases in the early period of isothermal holding and the rate of increase in peak stress slows with isothermal holding. During the rapid increase in the peak stress, the slope of the relationship shown in Fig. 4 in the early stage of isothermal holding is significantly higher than that in the later stage. Therefore, the peak stress appears to saturate in a range of stress that is considerably lower than the theoretical strength of the interface with full contact and no deformation.

Evolution of peak stress of the interface with isothermal holding of specimens with 14–20% initial compressive strain.
From Fig. 4, the peak stress of a specimen under an as-compressed condition with a higher initial compressive strain is lower than that of a specimen with a lower initial compressive strain. In the early stage, the peak stress of a specimen with higher initial compressive strain remains below that of a specimen with a lower initial compressive strain. However, a slightly steeper slope of the relationship in the early stage can be observed for a specimen with a higher initial compressive strain. Figure 5 shows the relationship between the initial compressive strain and the peak stress of the specimen under an as-compressed condition and specimens subjected to isothermal holding for 200 and 10000 ps. It can be seen that the peak stress decreases as initial compressive strain increased in the early stage of isothermal holding under an as-compressed condition and in case of isothermal holding for 200 ps. In the later stage of isothermal holding, on the other hand, the peak stress slightly increases with increasing initial compressive strain, showing less dependence on the initial compressive strain than that in the early stage. The results in Fig. 5 shows that the isothermal holding required for the early stage is longer for a specimen with a higher initial compressive strain. Moreover, the initial compressive strain appears to reduce the intrinsic strength of the interface in the early stage.

Relationship between initial compressive strain and peak stress of specimen under an as-compressed condition and with 200 and 10000 ps isothermal holding.
Figure 6 shows the relationship between the isothermal holding time and potential energy per atom, which was calculated using all atoms in the system, of the specimens with initial compressive strains of 14–20%. The potential energy is relatively high under an as-compressed condition and tends to continuously decrease with isothermal holding. Moreover, the decrease in potential energy appears to be a logarithmical function of isothermal holding time; thus the potential energy initially rapidly decreases then the decrease gradually slows with increasing isothermal holding time.
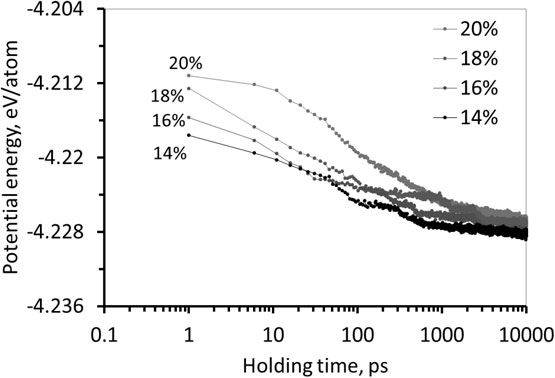
Potential energy per atoms of specimens with initial compressive strain of 14–20% plotted as a function of isothermal holding.
According to the relatively high potential energy under as-compressed condition, it can be implied that the deformation induced by the compression introduces a large amount of excess energy to the interface and its adjacent microstructure, so the atoms near the interface accommodate in highly unstable arrangement. Therefore, the difference in potential energy between an as-compressed and well-arranged condition of atoms in the system must play an important role as a thermodynamic driving force, $\Delta E$, for the rearrangement of atoms near the interface.
The decrease in potential energies from an as-compressed condition to isothermal holding of 10000 ps of all atoms in specimen and only atoms around the interface, which was calculated in small equal amount of atoms on both iron and nickel sides, was plotted as a function of initial compressive strain in Fig. 7. The decreases in potential energy of all atoms and interface increase with increasing initial compressive strain. Accordingly, the deformation induced by the compression potentially provides a driving force for the atomic rearrangement in the early stage of the isothermal holding, bringing about the increase in peak stress. The amount of driving force should affect a rate of the evolution of the peak stress, in the other word, slopes of relationship in Fig. 4 in the early stage. From the experiment of the solid-state bonding between steel and nickel in our previous study6), the evolution of strength per contact area in the first stage is also higher in the specimen with higher initial compressive strain. Subsequently, the strength per contact area seems to saturate at relatively the same value after rapid increase in the first stage irrelevant to the initial compressive strain. Therefore, the simulation results seem to be in good agreement with the evolution of interfacial strength of experiment6). The effect of deformation can also be observed in recovery and recrystallization process of deformed metals11–13). The deformation stored energy provides driving pressure for the recovery process and activates more homogeneous and heterogeneous nucleation for the recrystallization process.

Relationship between initial compressive strain and the decrease in potential energy per atoms from as-compressed condition to 10000 ps isothermal holding of all atoms in specimen and specific atoms near the interface.
Comparing with the decrease in potential energy of all atoms, since the decrease in potential energy of atoms near the interface is more significant and more sensitive to the initial compressive strain, it can be understood that the rearrangement occurs mainly at the atoms adjacent to the interface. Moreover, the deviation of calculation of decrease in potential energy of atoms near the interface is significantly higher than that of all atoms, because atomic movement near the interface is more active.
4.2 Interface fractureFigures 8(a) and 8(c) show macroscopic views of crystal structure on both bcc iron and fcc nickel sides of specimens with an initial compressive strain of 20% under as-compressed condition and isothermal holding of 1000 ps, respectively. Under an as-compressed condition, severe distortion of the crystal structure can be observed on both bcc iron and fcc nickel sides, as shown in Fig. 8(a). On the iron side, original lattice is divided by compression into several tiny grains scattering near the interface with slightly tilted orientation from the original direction. Also, thin layers of disordered atoms can be observed near the interface. In contrast to the iron side, the deformation accommodates throughout the lattice of fcc nickel with dislocation distribution. From Fig. 8(c), the distorted small grains near the interface on bcc iron side are agglomerated by nearby bigger lattices and the relaxation can be apparently observed on the nickel side after isothermal holding of 1000 ps. Note that the increase in contact area fraction of the interface from as-compressed condition to isothermal holding of 10000 ps is insignificant.

(a) and (c) are the macroscopic views near the interface of the specimens under an as-compressed condition and isothermal holding of 1000 ps with 20% initial compressive strain. (b) and (d) are stress analyzed macroscopic views at fracture stress of specimens under an as-compressed condition and isothermal holding of 1000 ps, respectively.
Both the specimens under an as-compressed condition and isothermal holding of 1000 ps were undergone a uniaxial tensile loading and the stress analysis in z axis of each atom at the ultimate value of stress during the loading was calculated and illustrated in Figs. 8(b) and 8(d), respectively. At the interface line, the stress of specimen with isothermal holding of 1000 ps tends to uniformly distribute across the interface, in contrast to that of specimen under as-compressed condition, whose stress is partly concentrated near the voids' tip at disordered layer. Therefore, the strength at the interface at atomic scale tends to increase with isothermal holding through atomic rearrangement, driven by the stored energy from deformation. Also, more energy dissipation at the adjacent lattices was found in the specimen with 1000 ps isothermal holding than that of specimen under as-compressed condition, so that the total energy dissipation seems to comprise of not only strength of the interface at atomic scale but also the energy dissipation around the interface in nanoscale.
The illustrations of stress analysis at the ultimate value of stress during the loading of specimens under an as-compressed condition with initial compressive strains of 14 and 18% are shown in Fig. 9. It can be seen that the stress of atoms of the specimen with higher initial compressive strain were restrained within small distorted grain adjacent to the interface in the specimen with higher initial compressive strain. Therefore, the atomic arrangement at the interface line and near the interface induced by severe deformation causes improvement in peak stress of specimen with short isothermal holding. Note that a range of initial compressive strains between 14–20% in this study was chosen, because the contact area fraction in the simulation should be relatively equivalent to the experiment in our previous study6).

(a) and (c) macroscopic views near the interface under an as-compressed condition of specimens with initial compressive strain of 14 and 18%, respectively. (b) and (d) stress analyzed macroscopic views at fracture stress of interface under an as-compressed condition of specimens with initial compressive strain of 14 and 18%, respectively.
Figure 10 shows the illustrations of the atomic arrangement at the interface on both bcc iron and fcc nickel sides of specimen with an initial compressive strain of 16% under as-compressed condition, isothermal holding of 30 ps and 200 ps, where the intrinsic strength of the interface continuously increases in the early stage. In order to determine whether am atom accommodates in each crystal structures, a computational method so-called dislocation extraction algorithm (DXA) was utilized by calculation of the burgers circuit and numbers of nearest neighboring atoms method14–16). The atoms, which is surrounded by white cloud, is considered not to accommodate in bcc crystal structure in case of bcc analysis, as shown in Fig. 10(a)–(c), and fcc crystal structure in case of fcc analysis, as shown in Fig. 10(d)–(f). The results show that thin layers of disordered atoms, ranging to a few angstroms, usually exist at the interface between bcc iron and fcc nickel sides. The thickness of the disordered atoms layer is relatively thick under as-compressed condition and tends to become thinner with the isothermal holding on both bcc iron and fcc nickel sides. Subsequently, very thin layer of disordered atoms on the iron side remains at the interface, however a number of nickel atoms still has not been rearranged into perfect fcc crystal structure. It appears that some nickel atoms near the interface accommodate in bcc crystal structure of iron and they are continuously rearranged to gain continuity between the bcc crystal structure on iron side and fcc crystal structure on the nickel side with the isothermal holding.

Illustrations of atomic arrangement at the interface of specimen with initial compressive strain of 16% analyzed by fcc structure algorithm for (a)–(c) and bcc structure algorithm for (d)–(f) under as-compressed condition, isothermal holding of 30 ps and 200 ps, respectively.
Figure 11 shows the relationship between isothermal holding time and a number of disordered atoms of both bcc iron and fcc nickel sides. It can be seen that a number of disordered atoms is extremely high under an as-compressed condition and tends to consistently decrease as isothermal holding increased, then the decrease halted and saturated. The decrease in number of disordered atoms involves two stages: the early and later stages. From Fig. 11, the amount of disordered atoms of a specimen with higher initial compressive strain is higher than that of a specimen with lower initial compressive strain. The isothermal holding required for the early stage was found to be longer in the specimen with higher initial compressive strain and this is comparably relevant to the early stage of the evolution of peak stress, as shown in Fig. 4. As the stress reached the ultimate value, the crack was found to initiate within thick layer of disordered atom in case of specimens with short isothermal holding, subsequently the fracture rapidly propagated through the layer of disordered atoms. On the contrary, the crack tended to initiate near the crack tips for the specimens with relatively long isothermal holding, still the fractures propagate through the layer of disordered atoms. Therefore, the layer of disordered atoms at the interface is considered the weak point of the specimen. Accordingly, the decrease in numbers of disordered atoms by short-range atomic rearrangement seems to play an important parts in evolution of peak stress in the early stage of isothermal holding. In contrast to the early stage, the decrease in disordered atoms in the later stage is insignificant, due to the incoherency between lattice plane between bcc iron and fcc nickel. As a results, the interface was still be the weak point of the specimen compared to the body of the lattices.

A number of disordered atoms plotted as a function of isothermal holding of specimens with initial compressive strain of 14–20%.
Referring to our previous study6), the deformation induced by the compression is deteriorating to the strength per contact area in the first stage by limitation of plastic energy dissipation at the microstructure near the interface in microscopic scale, in the meantime, the intrinsic strength of the interface is also detrimentally affected by the deformation from introducing a number of defects to the interface in atomic scale.
In this study, the atomistic simulation of solid-state bonding between dissimilar metals of iron and nickel atoms with varied compressive deformation were investigated. The intrinsic strength of the interface was evaluated by uniaxial tensile loading in MD simulation. The following conclusions were drawn in this study: