2018 Volume 59 Issue 5 Pages 741-746
2018 Volume 59 Issue 5 Pages 741-746
Mg–V–Cr alloys can be considered as alternatives to the Ti–V–Cr BCC (body-centered cubic) alloys for hydrogen storage at room temperature, but their synthesis has not been successful so far because Mg is immiscible in V and Cr. In this study, the first Mg–V–Cr BCC alloys were synthesized from elemental powders by severe plastic deformation via the high-pressure torsion (HPT) method as well as by high-energy ball milling. Structural homogeneity, thermal stability and hydrogen storage at room temperature were dependent on the composition and the best performances were achieved for the MgVCr composition with the maximum configurational entropy.
Despite significance of hydrogen as a clean fuel, the safe and high-density storage of hydrogen is a main challenge.1) Hydride-forming metals such as Mg, Ti, V and La are potential candidates for reversible, safe and high-density storage of hydrogen.2) However, since these metals produce stable hydrides which do not desorb hydrogen without heating, development of binary intermetallics such as LaNi5, TiFe and TiMn2 or ternary alloys such as Ti–V–Cr alloys with the BCC (body-centered cubic) structure has been a major strategy for hydrogen storage at room temperature.3–8)
Similar to the Ti–V–Cr BCC alloys, the Mg–V-based alloys such as Mg–V–Ni,9,10) Mg–V–Co,9) Mg–V–Cu,9) Mg–V–Ca,11) Mg–V–Sn10) and Mg–V–Pd10) were investigated as possible hydrogen storage materials. However, there are few attempts to synthesize the Mg–V–Cr BCC alloys as alternatives to the Ti–V–Cr BCC alloys, because Mg is totally immiscible in V and Cr.12) Therefore, developing new synthesizing methods is a key issue to use the Mg–V–Cr alloys for hydrogen storage. Mechanical alloying via high-energy ball milling2,3) or severe plastic deformation (SPD)13,14) can be considered as a possible route to synthesize the Mg–V–Cr alloys.
Recently, application of SPD through the high-pressure torsion (HPT) process15,16) showed a high potential to synthesize new composites,17,18) intermetallics,19,20) alloys21,22) and hydrogen storage materials23) even in the immiscible Mg-based systems such as Mg–Ti,24) Mg–Zr,25) Mg–V10) and Mg–Ni–Pd.26) The HPT method is not only a powerful route to synthesize bulk samples from powders27,28) but also an effective method to enhance the kinetics,27) activation4) and long-term performance30) of hydrogen storage materials.
In this study, the first Mg–V–Cr BCC alloys with different compositions are synthesized from the elemental powders using the HPT method and their crystal structure, microsctructure, hydrogen storage properties and thermal stability are examined. High-energy ball milling is also used as a synthesis route.
MgH2, V and Cr powders with <44 µm particle sizes and >99 mass% purities were mixed to produce MgV, Mg2VCr, MgV2Cr and MgVCr compositions. The powder mixtures were homogenized by low-energy ball milling under an argon atmosphere for 100 min with a rotation speed of 200 rpm using the Al2O3 balls and vial with a ball-to-powder mass ratio of 2:1. The powders were further processed between two HPT anvils15,16) under 3 GPa for N = 0 (mere compression), 10, 100 and 1200 turns (shear strains up to 70,000 which was technically the maximum strain that could be achieved in our facility) with an anvil rotation speed of 1 rpm to produce discs with 14 mm diameter and 0.75 mm thickness. Because of the thermodynamic immiscibility of Mg in V and Cr,12) the applied shear strains in this study were two orders of magnitude larger than those used for normal metal processing.31)
In addition to the HPT processing, an alloy with the MgVCr composition (which showed a good chemical homogeneity and stability after HPT processing) was synthesized from the MgH2, V and Cr powders by high-energy ball milling in a planetary mill using a stainless steel vial with an internal volume of 250 cm3. High-energy ball milling was performed for 24 h with a ball-to-powder mass ratio of 40:1 (25 chromium steel balls with diameters of 10 and 8 mm) and a rotational speed of 600 rpm. The vial was filled with 3 MPa of hydrogen (99.999%) during the milling process to minimize the oxidation. The powder mixture after high-energy ball milling was also processed by HPT under 3 GPa for N = 50 turns.
The processed samples were examined using (i) X-ray diffraction (XRD) analysis using the Cu Kα radiation, (ii) energy dispersive X-ray spectroscopy (EDS) in a scanning electron microscope (SEM) at mid-radius of discs under 15 kV, (iii) transmission electron microscopy (TEM) and scanning-transmission electron microscopy (STEM) under 300 kV, (iv) hydrogen pressure-composition-temperature (PCT) isotherms using a Sieverts-type apparatus on a disc crushed in argon at 303 K, and (v) XRD analysis in air after annealing at 423 and 573 K for 1 h and after PCT. Note that the samples were evacuated for 2 h at 303 K before PCT, after PCT and between the PCT cycles. The foils for TEM and STEM were prepared by focused ion beam (FIB) method at 6 mm away from the disc center.
To examine the elemental distribution and BCC-phase formation, SEM-EDS, XRD and STEM-EDS analyses were conducted after various turns, as shown in Fig. 1–3. SEM-EDS analyses show that the three elements gradually dissolve in each other with increasing the number of HPT turns. The elements entirely mix at the micrometer level in MgV2Cr and MgVCr after 1200 turns, while the distribution of elements is not uniform in MgV and Mg2VCr even after 1200 turns. XRD analyses also confirm that single BCC phases with the lattice parameters of a = 0.294 nm and 0.295 nm are formed in MgV2Cr and MgVCr, respectively. However, the peaks of the starting powders still co-present with the new BCC phases in MgV and Mg2VCr even after 1200 turns. STEM-EDS analyses after 1200 turns confirm that the distribution of elements is not uniform at the nanometer level even for MgV2Cr and MgVCr. Therefore, larger numbers of HPT turns are required to achieve a perfect elemental distribution because of thermodynamic immiscibility,10,23,24) but increasing the number of turns is not technically possible in our facility because of damages to the HPT anvils.
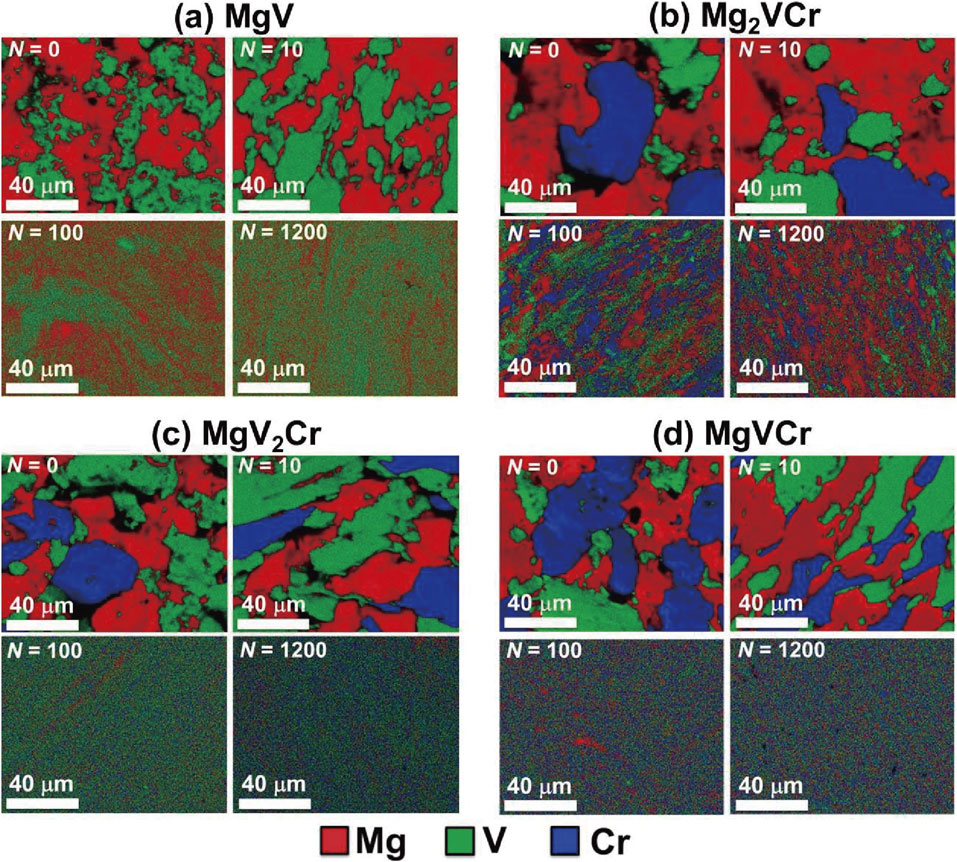
SEM-EDS mappings for (a) MgV, (b) Mg2VCr, (c) MgV2Cr and (d) MgVCr after various HPT turns.
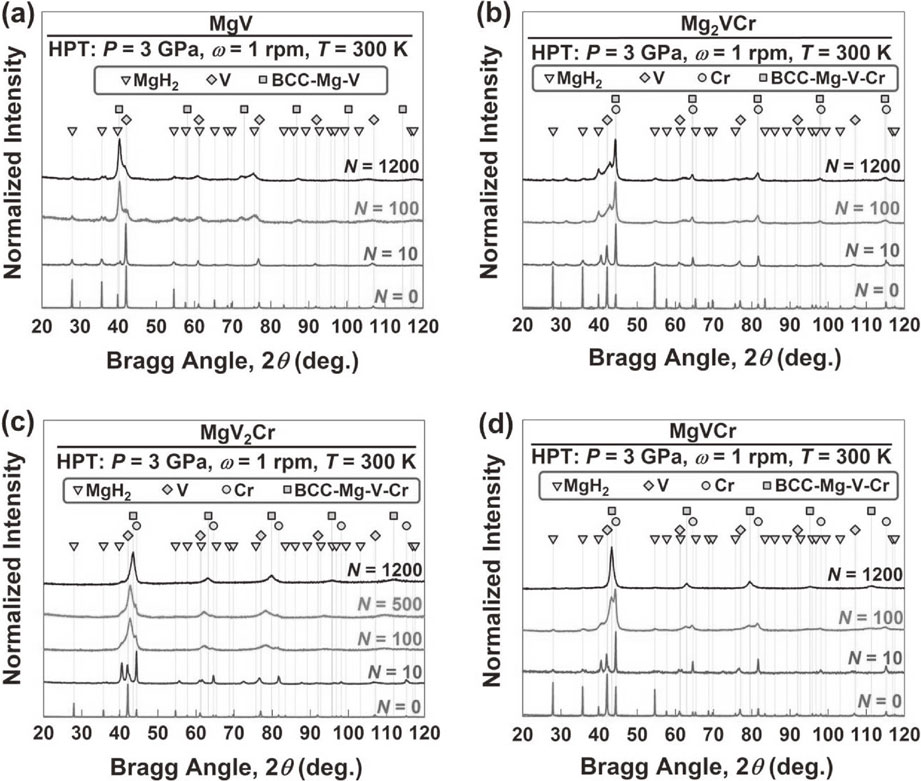
XRD profiles for (a) MgV, (b) Mg2VCr, (c) MgV2Cr and (d) MgVCr after various HPT turns.
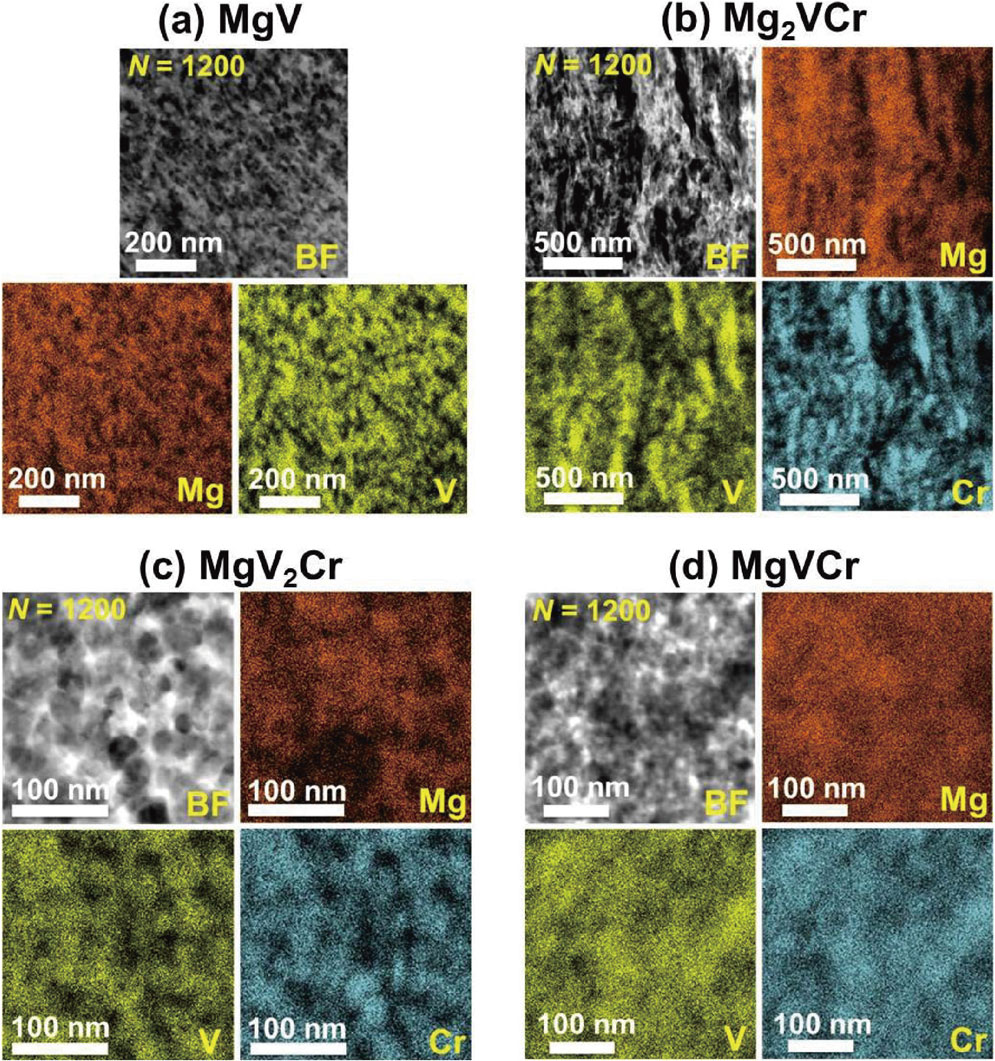
STEM-EDS mappings for (a) MgV, (b) Mg2VCr, (c) MgV2Cr and (d) MgVCr after 1200 HPT turns.
For further examination of the BCC phases and their nanostructure, the samples after processing for 1200 turns were examined by TEM, as shown in Fig. 4 for (a)–(c) MgV2Cr and (d)–(i) MgVCr. Bright-field and dark-field images show that nanograins with average sizes of 19 ± 6 nm for MgV2Cr and 18 ± 6 nm for MgVCr are formed. These grain sizes are smaller than those in many SPD-processed materials,15–17) but are comparable with those reported in HPT-processed immiscible and composite systems17–22) as well as in HPT-processed Mg-based intermetallics.32) The selected-area electron diffraction (SAED) results with perfect ring patterns taken from regions with 200 nm diameter confirm that the majority of nanograins have the BCC structure in agreement with the XRD profiles, although a few diffracted beams corresponding to the initial powders are still visible in Figs. 4(c) and (f). In addition to the formation of nanograined BCC phase, which can be further confirmed by (g) high-resolution TEM and (h) fast Fourier transform (FFT) analyses in Fig. 4, the edge dislocations are also visible in nanograins, as shown in Fig. 4(i) by inverse FFT. Although several studies suggested that the formation of lattice defects by HPT is effective to enhance the hydrogenation kinetics in Mg-based alloys,29,30,32) it was reported that the lattice defects can destroy the hydrogen storage properties in the Ti–V BCC alloys33) as well as in the HPT-processed Ti–V–Cr alloys.34) Therefore, it is essential to examine the hydrogen storage performance of the Mg–V–Cr BCC alloys after HPT processing.
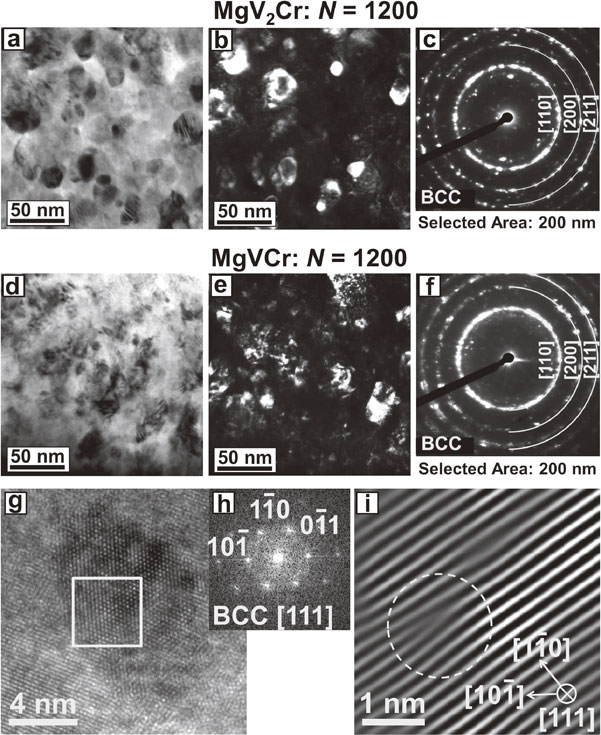
TEM (a), (g) bright-field images, (b), (h) dark-field images, (c), (i) SAED pattern, (d) high-resolution images, (e) FFT difractogram from the square area in (d), and (f) reconstructed lattice image of dislocation with inverse FFT for (a)–(c) MgV2Cr and (d)–(i) MgVCr processed by 1200 HPT turns.
To examine the hydrogen storage performance, PCT analyses were conducted at room temperature, as shown in Figs. 5(a), (b), and the materials after PCT analyses were quickly examined by XRD, as shown in Figs. 5(c), (d) for (a), (c) MgV2Cr and (b), (d) MgVCr. At room temperature, MgV2Cr and MgVCr absorb ∼0.9 mass% of hydrogen with ∼0.4 mass% reversibility. XRD analyses confirm that the hydrogen storage in MgV2Cr occurs in the forms of MgH2 and a VH-like hydride (see the peak indicated by an arrow in Fig. 5(c)), which indicate that the BCC phase decomposes during PCT analyses. The formation of this stable VH-like hydride should be the reason for the jump in hydrogen content at low pressures in Fig. 5(a). However, the hydrogen storage in MgVCr should have occurred in the form of a hydride without appreciable structural change because no MgH2, VH and Cr but only the BCC phase is detected in this alloy after PCT analyses. Therefore, MgVCr shows better phase stability for hydrogen storage at room temperature among the selected compositions. It should be noted that no XRD peak shift corresponding to the residual hydrogen in the BCC MgVCr alloy is detected in Fig. 5(d) after PCT measurement, indicating that the majority of residual hydrogen is desorbed during the post-PCT evacuation.
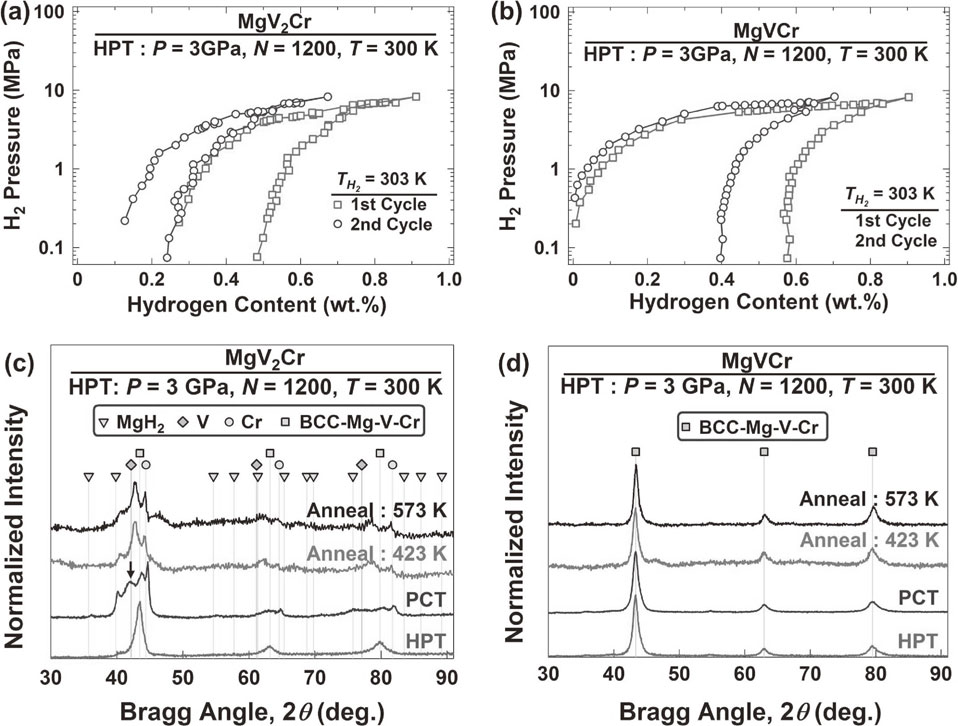
(a), (b) Hydrogen PCT isotherms at 303 K and (c), (d) XRD profiles after HPT followed by PCT at 303 K and annealing at 423 and 573 K for (a), (c) MgV2Cr and (b), (d) MgVCr processed by 1200 HPT turns.
To investigate the phase stability, XRD analyses were performed after HPT for 1200 turns followed by annealing at 423 and 573 K for 1 h, as shown in Fig. 5 for (c) MgV2Cr and (d) MgVCr. While the BCC phase in MgV2Cr disappears by annealing, the BCC-MgVCr phase remains stable even after annealing at 573 K. Although different parameters such as microstructure, diffusivity and heat of mixing influence the stability of phases with positive heat of mixing, the stability of MgVCr (which has similar structural and microstructural features to MgV2Cr) should be due to its higher configurational entropy as −R ln(1/3) (R: gas constant).35) The thermal stability results in Figs. 5(c, d) explain well the phase stabilities after hydrogen storage.
To examine the formation of Mg–V–Cr BCC alloys by other synthesizing methods, the MgVCr composition, which exhibited the best homogeneity and phase stability after HPT processing, was synthesized from the MgH2, V and Cr powders by high-energy ball milling. The ball milling was selected because numerous publications indicated the potential of the method for synthesizing hydrogen storage materials,9,11,36,37) for enhancement of hydrogen storage properties2,3,38,39) and for possible applications in industry.40–42) XRD profiles before ball milling, after ball milling for 24 h and after ball milling followed by HPT processing for 50 turns are shown in Fig. 6. The XRD profiles confirm that the initial powder mixture transforms mainly to a BCC phase after high-energy ball milling, although small peaks for the starting powders are still visible at 27.9° and 35.7°. These small peaks are less visible after additional HPT processing for 50 turns, indicating a combination of the two methods is more effective for better synthesis. The current results confirm that the formation of the Mg–V–Cr BCC alloys is not limited to the HPT method and other synthesizing techniques such as high-energy ball milling can be used to synthesize the alloys, although limited hydrogen storage capacity is still a concern.
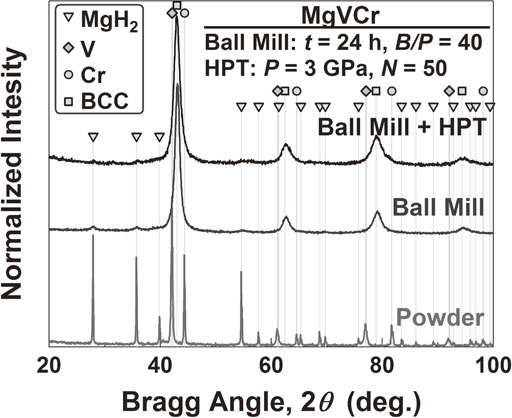
XRD profiles before and after high-energy ball milling for 24 h and after high-energy ball milling followed by HPT processing for 50 turns.
A question arises from the current study. Why the hydrogen storage capacity of MgVCr is lower than the expected capacity for the BCC-based hydrogen storage alloys (2.3 mass% and 4.6 mass% for MgVCrH3 and MgVCrH6 with metal-to-hydrogen ratios of 1 and 2, respectively). Because of similarity between the crystal structures of the Mg–V–Cr BCC alloys and Ti–V–Cr BCC alloys, room temperature hydrogen storage in MgVCr is expected. However, the room temperature hydrogen storage of Ti–V–Cr alloys is affected by activation and complete hydrogen storage does not occur without a thermal6) or mechanical34) activation treatment. Therefore, to achieve the maximum hydrogen storage in the Mg–V–Cr alloys, an activation process should be developed. The activation process of Mg–V–Cr alloys seems even more complicated than the Ti-based hydrogen storage alloys, and the authors’ attempt to find an appropriate activation process has not been successful so far. Moreover, although the nanograins and lattice defects can usually enhance the hydrogenation kinetics28–30) and activation,4) they can destroy the hydrogen storage capacity in the Ti–V-based BCC alloys.32,34) Therefore, another reason for low hydrogen storage capacity of Mg–V–Cr alloys when compared to other room temperature hydrogen storage materials such as LaNi5,3) TiFe,4) TiMn25) and Ti–V–Cr6–8) can be due to the effect of lattice defect which are formed during mechanical synthesizing by HPT. Development of an appropriate heat treatment to recover the lattice defects and enhance the activation is currently under investigation by the authors.
In in this study, the Mg–V–Cr BCC alloys were synthesized by high-pressure torsion and high-energy ball milling for possible hydrogen storage application at room temperature. The synthesized materials could absorb hydrogen at room temperature, but they exhibited rather low hydrogen storage capacity as 0.9 mass%, probably due to the activation issues. An alloy with the MgVCr composition exhibited the best homogeneity and stability during both thermal treatment and hydrogen storage.
K.E. thanks the MEXT, Japan, for a Grant-in-Aid for Scientific Research (B) (No. 16H04539). This study was supported in part by the MEXT, Japan, through a Grant-in-Aid for Scientific Research (S) (No. 26220909). The HPT process was carried out at IRC-GSAM, Kyushu University, Japan.