2018 Volume 59 Issue 8 Pages 1225-1232
2018 Volume 59 Issue 8 Pages 1225-1232
A p-type Bi0.3Sb1.7Te3.0 thermoelectric semiconductor, which has a high dimensionless figure of merit (ZT), was prepared by milling at various milling rotational speeds. The milled Bi0.3Sb1.7Te3.0 powder changed from a partially alloyed state to a completely alloyed one with increasing rotational speed.
The thermoelectric properties of Bi0.3Sb1.7Te3.0 sintered disks differed depending on the milling rotational speed. The minimum electrical conductivity σ, thermal conductivity κ, and maximum Seebeck coefficient α at the rotational speed at which the transition from PIES (pulverized intermixed elements sintering) process to MA (mechanical alloying) followed by HP (hot pressing) occurred.
The ZT varied with changes in rotational speed, with a maximum value were obtained. This study have shown that the thermoelectric properties of materials prepared by powder metallurgy used milling were significantly affected by the milling energy, i.e., the rotational speed.
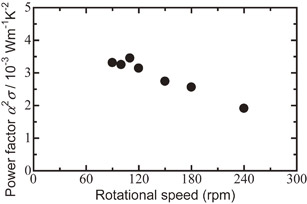
Fig. 9 Relationship between power factor α2σ at room temperature and milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disk followed by hot pressed at 623 K.
Thermoelectric materials are attracting attention because they have potential applications in energy conversion and saving, using recovered exhaust waste heat.1,2) Energy conversion systems based on thermoelectric materials have many advantages. They are noise free, virtually maintenance free, highly economical for long-term operations, and environmentally friendly,1,2) that is, thermoelectric materials are recognized as eco-materials.1–3) Recently, many thermoelectric materials, and modules and power generation systems based on them, have been studied.4–7)
Bi2Te3–Sb2Te3 solid solutions are the one of the best p-type thermoelectric semiconductors near room temperature, and are widely used for refrigerators and experimental generators.2,8)
The Bi2Te3–Sb2Te3 solid solution has a rhombohedral crystal structure with the R3m space group, and anisotropic physical and thermoelectric properties.8) Materials based on Bi2Te3–Sb2Te3 solid solutions are anisotropic.8,9) The anisotropies of the electrical and phonon thermal conductivities of Bi2Te3 and its related materials are estimated to be σ∥/σ⊥ ≈ 3 and κ∥/κ⊥ ≈ 0.47, respectively, for the values parallel and perpendicular to the c basal plane; however, the Seebeck coefficient is isotropic.9)
The performance of a thermoelectric semiconductor is defined by the dimensionless figure of merit ZT:
| \begin{equation} ZT = \alpha ^{2}\sigma \kappa ^{ - 1}T \end{equation} | (1) |
| \begin{equation} \kappa _{\text{total}} = \kappa _{\text{phonon}} + \kappa _{\text{carrier}} = \kappa _{\text{phonon}} + L\sigma T \end{equation} | (2) |
| \begin{equation} L = ( \pi k_{\text{B}} )^{2}( 3e^{2} )^{ - 1} = 2.45 \times 10^{ - 8}\,\text{WS}^{ - 1}\text{K}^{ - 2} \end{equation} | (3) |
Bi2Te3–Sb2Te3 solid solution materials are generally prepared using melt growth methods such as zone melting or the Bridgman method.12) The solid solution effect reduces the phonon thermal conductivity, depending on the mixing ratio, of thermoelectric materials such as has been reported for Si–Ge.9,13,14) Thus the p-type BixSb2−xTe3.0 have a high dimensionless figure of merit of ZT at room temperature.2) Especially, the composition Bi0.5Sb1.5Te3.0 provides the best thermoelectric material prepared using a melt growth method because the phonon thermal conductivity is minimized by the solid solution effect.2) However, the reduction in the phonon thermal conductivity as a result of the fine-grain effect in mechanical alloying followed by hot pressing (MA-HP) is much greater than the reduction arising from the solid solution effect in the melt growth method.15) The maximum ZT of the Bi2Te3–Sb2Te3 solid solution obtained using MA-HP was higher than that of Bi0.5Sb1.5Te3.0 obtained using the melt growth method.15) Bi0.3Sb1.7Te3.0 prepared using MA-HP showed a maximum ZT of 1.16 at 367 K.15) In previous study which was experiment of depends of ZT on the Bi content in the MA–HP process. As a result, the remarkable maximum ZT composition was sifted from Bi0.5Sb1.5Te3.0 to Bi0.3Sb1.7Te3.0.15)
In recent years, many methods have been developed for the preparation of samples with fine-grained structures using MA.8,16–20) Hot- and cold-pressing processes combined with MA give fine and high-density samples.21–26) Spark plasma sintering combined with MA is frequently used because the preparation process is rapid.27–32) Dopant effects and the preparation conditions such as milling time or pressing pressure have been widely studied.33–36) Milling is not only used for Bi–Sb–Te-based materials but also for other thermoelectric materials such as ZnSb,37) EuZn2Sb2,38) Al2Fe3Si3,39) Mg2Si0.75Sn0.25,40) and BiSeTe.41)
The ball-milling processes used in the preparation of thermoelectric materials can be categorized as low- and high-energy ball milling.20,42) The powder of pulverized and intermixed elements sintering (PIES) process defines as partial alloying and remained raw material after milling. On the other hand, the powder of mechanical alloying followed by hot pressing (MA-HP) process defines as completely alloying after milling.20,42) It is not clear whether PIES or MA-HP is better for preparing high-performance thermoelectric materials in the literature. Furthermore, the appropriate milling conditions for synthesizing thermoelectric materials have not yet been identified. For Bi2Te3, a higher temperature and milling energy both tend to produce large grains.43) So, optimum milling condition might be recognized that it can control grain-growth.43) The milling energy depends on the rotational speed and the ball-milling apparatus. If these conditions can be controlled, the low thermal conductivity and the high ZT are obtained.
In the present study, the effects of the proper milling rotational speed on the thermoelectric properties of Bi0.3Sb1.7Te3.0 by the structure observation such as XRD, SEM and elemental analysis, especially at the point of transition from a PIES process to an MA-HP process, were investigated in hopes that the results will be useful for the preparation process of all thermoelectric materials.
The stoichiometric amounts of Bi (99.999%), Sb (99.9999%), and Te (99.9999%) needed to produce Bi0.3Sb1.7Te3.0 which was indicated to give the maximum ZT prepared using MA-HP without additional dopants were weighed out.15) Only millimeter-scale grains of the raw materials with small surface areas were used, to suppress contamination by a surface oxide layer. The raw materials were placed in a stainless-steel vessel with milling balls made of a silicon nitride ceramic in a glove box filled with Ar gas. The ball diameter was 25 mm. The weight ratio of milling balls to the raw materials was more than 20:1. Milling was performed using a Fritch P-5 planetary ball mill (maximum revolution speed: 300 rpm, revolution : rotation = 1:−2.18) for 30 h.
The vessel was mounted on disks and rotated around its own axis. The raw materials were initially crushed by collisions with the milling balls moving in the vessel. The centrifugal force caused by rotation of the vessel and mounted disks increases in proportion to the square of the rotational speed. When the rotational speed is increased, pulverizing and intermixing of the elements of the raw materials caused by low-energy impact with the balls changes to element alloying caused by the collisions of high-energy balls.
The rotational speed was varied from 60 to 240 rpm. The milled powder was passed through a 150 µm diameter metallic sieve to remove any remaining raw materials. The milled powder that did not pass through the sieve was not sintered.
The milled powder that passed through the sieve was examined using differential thermal analysis (DTA; TG/DTA6300, Hitachi High-Tech Science Corporation) at a heating rate of 10 K min−1 under a helium gas flow, and X-ray diffraction (XRD; Rigaku Multiflex), using Cu Kα radiation, in the Bragg angle range 2θ = 20–80° with a step size of 0.1° and a step speed of 1.0 s, to confirm alloying of the milled powder.
The milled powder that passed through the sieve was sintered by HP at 623 K under a uniaxial pressure of 147 MPa in an Ar atmosphere.
Cylindrical sintered compacts of diameter 10 mm and height 9 mm were prepared. These compacts were cut into disks of height and diameter approximately 1 mm and 10 mm, respectively, to obtain isotropic disks. Approximately 1 mm of material was removed from the top and bottom of the sintered compact surface because it has been reported that the surface of a sintered compact has the (00·l) texture.15,44)
The section parallel to the top plane of the sintered disks were examined by XRD (Rigaku Multiflex), using Cu Kα radiation, in the Bragg angle range 2θ = 20–80° with a step size of 0.1° and a step speed of 1.0 s. The milled powder that did not pass through the sieve was not examined using XRD.
The surfaces of the sintered disks were polished and then slightly chemically etched. Al2O3 powder was used as polishing grits. The microstructures of the out-of-plane sintered disks were observed using scanning electron microscopy (SEM; JEOL JSM-6510A) to determine whether contamination had occurred, based on secondary electron imaging (SEI). Elemental analysis was performed at the same time.
The room-temperature Seebeck coefficients, electrical conductivities, and thermal conductivities of all the disks were determined, and their power factors α2σ and dimensionless figures of merit ZT were estimated. The Seebeck coefficient was determined using the constructed thermal contact method after calibration using SRM 3451,41,45,46) and the thermal conductivity was determined using the constructed static comparison method with quartz of thickness 1 mm, diameter 10 mm, and thermal conductivity 1.411 W m−1 K−1 at room temperature as a reference.2,41,47)
The electrical conductivity was measured by the four-point probe method using an electrical resistance system based on a 2182A/6220 instrument (Keithley Instruments, Inc.) The probe size was 1.0 mm and it was made of tungsten carbide. All measurements made using the electrical conductivity system were confirmed by ohmic contact.41)
The accuracy of the Seebeck coefficient was less than ±2%, and those of the electrical conductivity and thermal conductivity were both less than ±1%.41) The maximum figure of merit of Bi0.3Sb1.7Te3.0 at room temperature was determined, and the temperature dependences of the Seebeck coefficient and electrical conductivity from 300 to 573 K were determined using a ZEM-3 instrument (Advance-Riko). A Bi0.3Sb1.7Te3.0 sintered compact of dimensions 10 mm × 10 mm × 9 mm was obtained using MA-HP. A specimen of dimensions 3.0 mm × 3.0 mm × 8.0 mm was removed from the center of the sintered compact to eliminate the effects of anisotropy. The ZEM-3 instrument had an accuracy of less than ±7%; the dimensionless figure of merit ZT was estimated from the temperature dependences of the Seebeck coefficients and electrical conductivities obtained using the ZEM-3 instrument, and the thermal conductivity at room temperature was determined using the static comparison method.
Pulverization in the milling operation at a rotational speed equal to or less than 150 rpm was cataracting motion, but at rotational speeds above 150 rpm, the process was over critical mill speed. The milled powder was separated from remaining raw materials using a 150 µm diameter sieve. The powders obtained by milling at 60 to 80 rpm did not pass through the sieve and showed poor sinterability or could not be sintered under the present experimental conditions. Millimeter-scale grains of the raw materials remained in these powders. The powders obtained by milling at or above 90 rpm passed through the sieve. They were sintered and their thermoelectric properties were examined.
Figure 1 shows the XRD patterns of Bi0.3Sb1.7Te3.0 powders obtained by milling for 30 h. The circles, squares, triangles, and solid circles indicate Bi, Sb, and Te elements, and a Bi2Te3–Sb2Te3 solid solution, respectively. The powders obtained by milling at 60 to 80 rpm were not examined using XRD because raw materials remained in the milled powders and did not pass through the sieve. Milling of the raw materials at 90 rpm or above pulverized and intermixed the elements, and the obtained powders were examined using XRD. Bi and Sb elements were not detected separately because they were started to alloy in the solid solution formed by milling at 90 rpm. The alloying of Bi–Te and Sb–Te clearly increased with increasing rotational speed above 90 rpm, and separate Bi, Sb, and Te elements were not observed after milling at 150 rpm. The milling process changed from partial alloying of PIES to complete alloying of MA-HP with increasing rotational speed.

XRD patterns of Bi0.3Sb1.7Te3.0 powders milled at rotational speeds of 90, 110, 150, and 180 rpm. Circles, squares, triangles, and solid circles indicate Bi, Sb, and Te elements, and Bi2Te3–Sb2Te3 solid solution, respectively.
Figure 2 shows the DTA curves obtained for milling alloyed powders obtained at rotational speeds from 90 to 180 rpm. The first endothermic peak, at around 673 K, corresponds to the eutectic point of Te and Bi2Te3.48) The first peak indicates the remaining Te content, therefore its area decreased with increasing rotational speed because alloying progressed with increasing rotational speed. These results are consistent with the XRD patterns in Fig. 1. The second endothermic peak, at around 873 K, corresponds to the melting point of Bi0.3Sb1.7Te3.0.9)
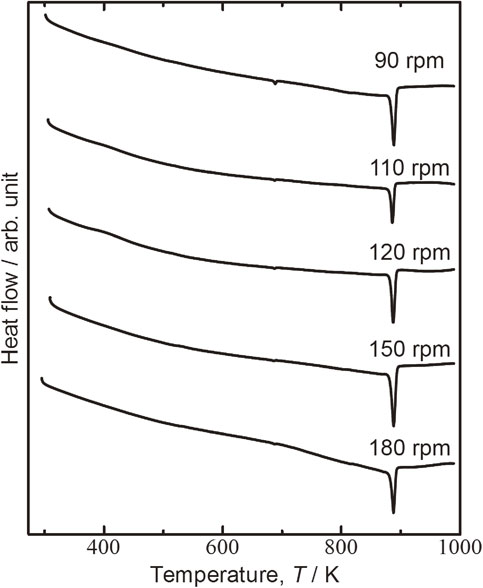
DTA curves of Bi0.3Sb1.7Te3.0 powders milled at rotational speeds of 90, 110, 150, and 180 rpm.
Figure 3 shows the XRD patterns of Bi0.3Sb1.7Te3.0 sintered disks milled at rotational speeds of 90, 110, 150, and 180 rpm, followed by HP at 623 K. The main hkl indexes show that the material is a Bi2Te3–Sb2Te3 solid solution. No peaks are observed from Bi, Sb, and Te elements, and other compounds. All the diffraction peaks indicate a single-phase, isotropic Bi2Te3–Sb2Te3 solid solution. The remaining unalloyed materials such as Bi, Sb, and Te elements disappeared and a homogeneous solid solution was formed during subsequent sintering.
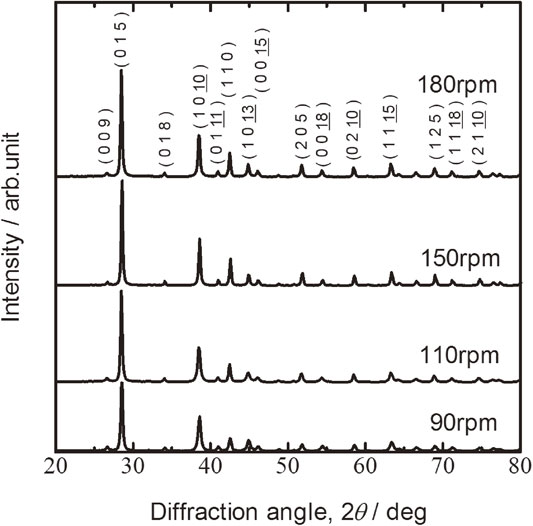
XRD patterns of Bi0.3Sb1.7Te3.0 sintered disks milled at rotational speeds of 90, 110, 150, and 180 rpm, followed by hot pressed at 623 K. Main hkl indexes show that the material is a Bi2Te3–Sb2Te3 solid solution.
A Bi2Te3–Sb2Te3 solid solution was formed by alloying of the remaining raw materials during sintering. This is consistent with the PIES method.42) The diffraction peak intensities increased with increasing rotational speed. HP can improve crystallization by increasing interdiffusion. Although Bi, Sb, and Te elements remained after milling at or below 110 rpm, all the milled powder was completely alloyed by HP.
Figure 4 shows SEM micrographs obtained by SEI, and elemental analysis for the corresponding areas in Fig. 5, for areas 001, 002, and 003 of Bi0.3Sb1.7Te3.0, milled at rotational speeds of (a) 90, (b) 110, and (c) 150 rpm, respectively. All samples were dense and had no pores. All the samples had fine grains of size less than 1 µm. The white particles are polishing grits. SEI and elemental analysis of the sample milled at a rotational speed of (a) 90 rpm, i.e., area 001 in Fig. 5, showed non-uniform dispersion of elements. The bright areas in the micrograph indicate a heavy element, suggesting Bi segregation. SEI of the sample milled at a rotational speed of (b) 110 rpm showed uniform elemental dispersion. SEI of the sample milled at a rotational speed of (c) 150 rpm showed that it contained many inclusions. Elemental analysis of area 002 in Fig. 5 indicated the presence of Fe, Ni, and Cr. This shows that contamination from the stainless-steel vessel occurred at a rotational speed of (c) 150 rpm. Elemental analysis of area 003 in Fig. 5 indicated the presence of Bi, Sb, and Te.
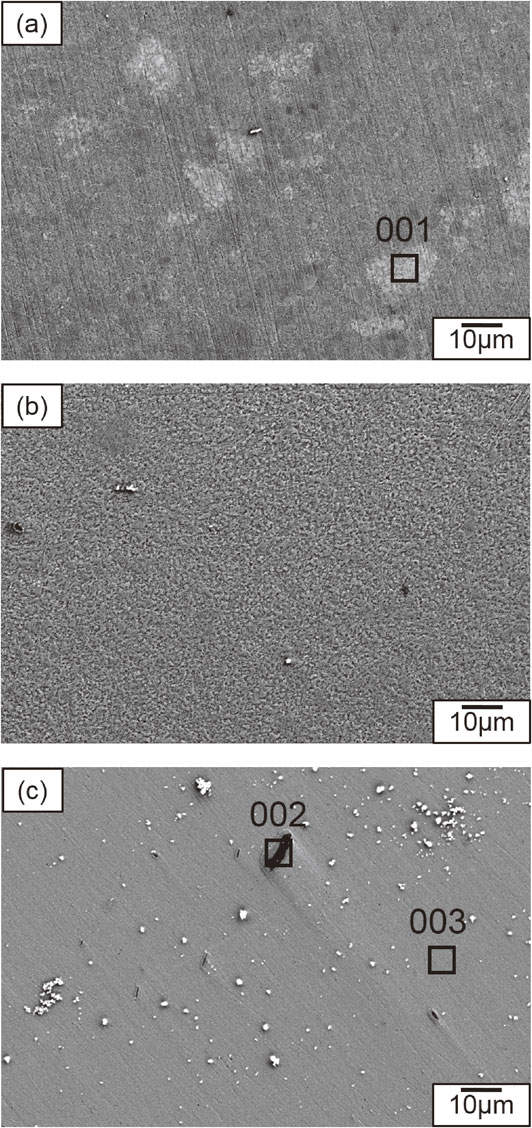
SEM micrographs and elemental analyses of corresponding areas for samples 001, 002, and 003 of Bi0.3Sb1.7Te3.0, milled at rotational speeds of (a) 90, (b) 110, and (c) 150 rpm followed by hot pressed at 623 K, respectively.

Elemental analyses of corresponding areas for samples 001, 002, and 003 of Bi0.3Sb1.7Te3.0, milled at rotational speeds of (a) 90, (b) 110, and (c) 150 rpm followed by hot pressed at 623 K, respectively.
Figure 6 shows the relationship between the electrical conductivity σ at room temperature and the milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disks hot pressed at 623 K.
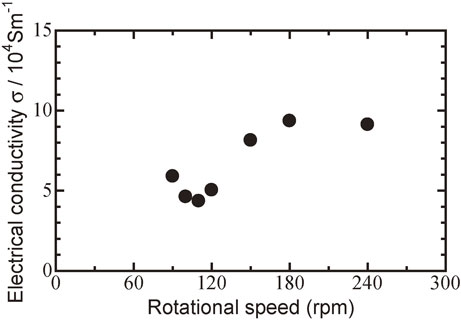
Relationship between electrical conductivity σ at room temperature and milling rotational speed (rpm) for Bi0.3Sb1.7Te3.0 sintered disks followed by hot pressed at 623 K.
The electrical conductivity of the Bi0.3Sb1.7Te3.0 sintered disks decreased with increasing rotational speed. The electrical conductivity of the sintered disk milled at 90 rpm was 5.90 × 104 S m−1, and it decreased when the rotational speed was increased to 110 rpm, as a result of improved alloying and interdiffusion. This trend showed relativity with the results of XRD peaks in Fig. 1, DTA curves in Fig. 2 and the SEM images in Fig. 4(a), (b), i.e., milling did not uniform at rotational speed below 110 rpm. As a result, small amounts of Bi, Te, and Sb elements, which were undetected by XRD (Fig. 3), remained in the sintered compacts milled at rotational speeds below 110 rpm. These elements could act as dopants in the Bi0.3Sb1.7Te3.0 solid solution, resulting in increased carrier concentrations below 110 rpm.
The minimum electrical conductivity, 4.36 × 104 S m−1, was observed for Bi0.3Sb1.7Te3.0 sintered disks milled at 110 rpm, indicating a transition between PIES and MA-HP. This is confirmed by Fig. 3, which shows uniform elemental dispersion on the surface in the out-of-plane direction.
In contrast, the electrical conductivity increased with increasing rotational speed above 110 rpm. The constituent elements of the stainless-steel vessel, e.g., Fe, Cr, and Ni, could contaminate Bi0.3Sb1.7Te3.0 and act as dopants, increasing the carrier concentration from the view of SEM images in Fig. 4(c) and elemental analysis at area 002 in Fig. 5. This effect would be greater at 180 rpm because the impacts of the milling balls could cause diffusion of Fe, Cr, and Ni from the stainless-steel vessel walls.49,50) At rotational speeds above 180 rpm, ball milling could become over critical mill speed, i.e., orbital milling, and the electrical conductivity became saturated at 9.13 × 104 S m−1. The reason for this saturation is that contamination with elements such as Fe, Cr, and Ni became stable because the frequency of collisions among high-energy milling balls, the stainless-steel wall, and raw materials reached a maximum, therefore the carrier concentration reached a maximum at rotational speeds above 180 rpm.
Figure 7 shows the relationships between the total, phonon, and carrier thermal conductivities, and the milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disks. The total thermal conductivity decreased with increasing milling rotational speed from 90 to 110 rpm. The thermal conductivity of the Bi0.3Sb1.7Te3.0 sintered disk milled at 110 rpm was 1.02 W m−1 K−1, which is the minimum total thermal conductivity at the transition between PIES and MA-HP.
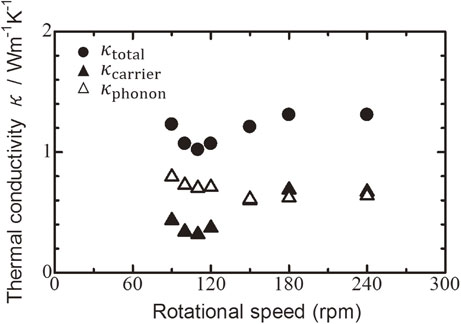
Relationships between total, phonon, and carrier thermal conductivities at room temperature, and milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disk followed by hot pressed at 623 K.
The total thermal conductivity increased with increasing milling rotational speed up to 180 rpm. The total thermal conductivity of sintered disks milled at or above 180 rpm became saturated at 1.31 W m−1 K−1.
The phonon and carrier thermal conductivities were estimated using eqs. (2) and (3). The phonon thermal conductivity decreased with increasing milling rotational speed from 90 to 150 rpm because of the fine-grain effect and increased alloying. The phonon thermal conductivities of sintered disks milled at rotational speeds above 150 rpm were constant. This is because the collision frequency reached a maximum because the high-energy ball milling became orbital ball milling, i.e., stable and periodic.
The carrier thermal conductivity decreased with increasing milling rotational speed from 90 to 110 rpm. This result followed with eq. (2), which means relationship of the between carrier thermal and electric conductivities.
Figure 8 shows the relationship between the Seebeck coefficient α and the milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disks. All the disks showed p-type conduction at room temperature. The Seebeck coefficient increased with increasing rotational speed from 90 to 110 rpm. The maximum Seebeck coefficient, 281 µV K−1, was obtained for the Bi0.3Sb1.7Te3.0 sintered disk milled at a rotational speed of 110 rpm, i.e., at the transition between PIES and MA-HP.
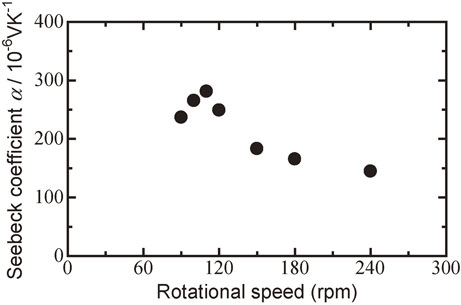
Relationship between Seebeck coefficient α at room temperature and milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disk followed by hot pressed at 623 K.
The Seebeck coefficient decreased with increasing milling rotational speed from 120 to 240 rpm. These results are consistent with those for the electrical conductivity because there is an inverse relationship between the Seebeck coefficient α and the electrical conductivity σ based on the carrier balance.51)
Figure 9 shows the relationship between the power factor α2σ and milling rotational speed for Bi0.3Sb1.7Te3.0. The power factor decreased with increasing rotational speed. The power factor of the Bi0.3Sb1.7Te3.0 sintered disk obtained at 110 rpm showed the maximum value, i.e., 3.45 × 10−3 W m−1 K−2. The power factor in the PIES range was larger than that for MA-HP, probably because the small amounts of remaining elements such as Bi, Sb, and Te acted as dopants.

Relationship between power factor α2σ at room temperature and milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disk followed by hot pressed at 623 K.
Figure 10 shows the relationship between the dimensionless figure of merit ZT and the milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disks. The ZT value increased with increasing rotational speed from 90 to 110 rpm because the thermal conductivity decreased, as shown in Fig. 6. The maximum ZT was 1.01, at 110 rpm. The ZT value decreased gradually with increasing milling rotational speed from 120 to 240 rpm. The range of transition from a PIES process to an MA-HP process was obtained the maximum ZT.
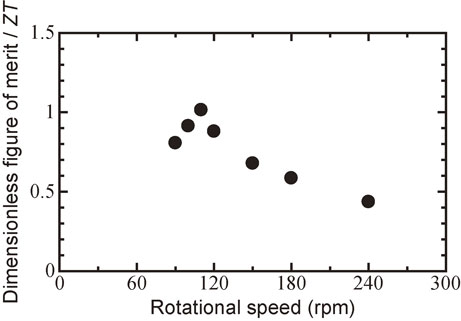
Plot of dimensionless figure of merit ZT at room temperature versus milling rotational speed for Bi0.3Sb1.7Te3.0 sintered disk followed by hot pressed at 623 K.
Figure 11 shows the Seebeck coefficient versus temperature plot for the sintered Bi0.3Sb1.7Te3.0 specimen milled at 110 rpm. The Seebeck coefficient increased slightly up to 367 K and then decreased above 367 K. The maximum Seebeck coefficient was 268 µV K−1 at 367 K.
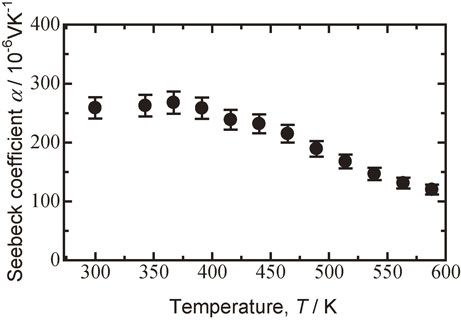
Plot of Seebeck coefficient versus temperature for Bi0.3Sb1.7Te3.0 sintered specimen milled at 110 rpm followed by hot pressed at 623 K.
Figure 12 shows a plot of the electrical conductivity of the sintered Bi0.3Sb1.7Te3.0 specimen milled at 110 rpm versus temperature. The electrical conductivity decreased with increasing temperature. Bi0.3Sb1.7Te3.0 synthesized using MA-HP had the conductive properties of a degenerate semiconductor.
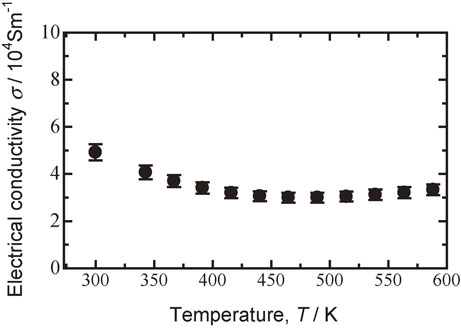
Plot of electrical conductivity versus temperature for Bi0.3Sb1.7Te3.0 sintered specimen milled at 110 rpm followed by hot pressed at 623 K.
Figure 13 shows a plot of the dimensionless figure of merit ZT for sintered Bi0.3Sb1.7Te3.0 milled at 110 rpm versus temperature at a fixed total thermal conductivity κtotal at room temperature. It was presumed that because of the Wiedemann–Franz law and high-temperature Umklapp process, the thermal conductivity would be approximately constant in this temperature range.47) The maximum ZT, i.e., 0.97, was obtained at 300 K.
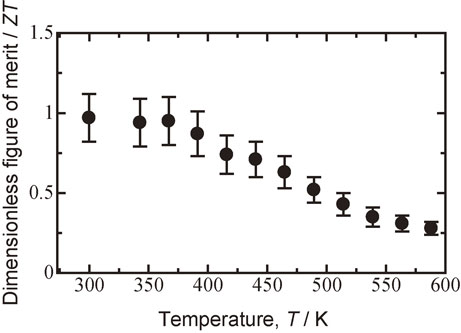
Plot of dimensionless figure of merit ZT of Bi0.3Sb1.7Te3.0 sintered specimen milled at 110 rpm followed by hot pressed at 623 K versus temperature at fixed total thermal conductivity κtotal at room temperature.
These results indicate that the thermoelectric properties, as well as the contamination, of powder metallurgically processed materials are significantly affected by the milling energy, in this case, the rotational speed of planetary ball milling.
In the present study, the dependences on the milling rotational speed of the thermoelectric properties of Bi0.3Sb1.7Te3.0 sintered disks were investigated. The conclusions were as follows.
These results indicate that in the range of transition from a PIES process to an MA-HP process was obtained the maximum ZT and the thermoelectric properties of materials prepared by powder metallurgy are significantly affected by the milling energy, i.e., the rotational speed. Contamination was related to the rotational speed and could affect the thermoelectric properties.
This work was supported by JSPS KAKENHI Grant Number JP17K06863 and the Sasakawa Scientific Research Grant from The Japan Science Society Grant Number 29-743. We thank Helen McPherson, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.