2019 Volume 60 Issue 1 Pages 165-168
2019 Volume 60 Issue 1 Pages 165-168
The effect of initial microstructures of low-carbon steel on austenite formation behavior during intercritical annealing was investigated. Three types of hot-rolled sheet specimens with different microstructures were used; specimen P consisting of ferrite and pearlite, specimen B consisting of bainitic structures, and specimen M consisting of fully martensitic structures. After the hot rolling, these specimens were cold-rolled, and subsequently heated to target temperature, and then water-quenched to room temperature. The martensite and/or bainite fraction corresponds to the fraction of austenite during intercritical annealing since the austenite transforms into martensite and/or bainite during the cooling process. The austenite fraction in specimen M was larger than that in specimens P and B below 730°C, whereas the order of specimens changed to P > B > M above 740°C. Below 730°C, austenite connected along the rolling direction was observed in specimens P and B, while the distribution of austenite in specimen M was uniform. In contrast, austenite was connected and elongated along the rolling direction in all the specimens above 740°C. The nucleation and growth of austenite can proceed under local equilibrium in specimens P and B, whereas that can proceed under paraequilibrium in specimen M below 730°C. Moreover, the austenite growth can progress under local equilibrium in all specimens above 740°C.
Low-carbon high-strength steel is used in automotive parts such as structural and reinforcing components. It is well known that the mechanical properties and formability of low-carbon steel are strongly dependent on the microstructure. However, it has been reported that the microstructure of low-carbon steel is complicated because several metallurgical phenomena occur simultaneously during annealing.1–4) Therefore, analysis of the microstructure of low-carbon steel is important to precisely control the mechanical properties and formability.
Several authors have studied the effect of initial microstructures on the microstructural evolution during annealing in low-carbon steel.5–7) For instance, Karmakar et al. reported7) that the effect of the initial microstructure on the microstructural evolution during annealing tends to be significant when the heating rate is rapid. In addition, we have demonstrated that progress of the recovery and recrystallization of ferrite during annealing is strongly dependent on the initial microstructure.8) In our previous study, it was revealed that the progress of recovery and recrystallization of ferrite is rapid when the initial microstructure consists of martensite, which leads to the acceleration of austenite formation at the early stage of intercritical annealing. The distribution of austenite has also been confirmed to be strongly dependent on the morphology of the recrystallized ferrite grains, irrespective of the cementite distribution before annealing. Thus, it is apparent from such previous studies that the initial microstructure is important to control the microstructural evolution during annealing. However, the effect of the initial microstructures on the austenite formation behavior at the latter stage of intercritical annealing has not yet been investigated. Thus, to achieve precise control of the microstructure, it is necessary to investigate the austenite formation behavior over a wide temperature range.
Therefore, the purpose of the present study is to investigate the effect of initial microstructures on the austenite formation behavior in low-carbon steel during rather high temperature intercritical annealing.
The chemical composition of the tested steel (in mass%) is 0.1C–2.0Mn, which is based on our previous study.8) The vacuum-melted ingots (approximately 100 × 100 × 500 mm) were rough rolled to a thickness of 30 mm. The rough rolled steels were hot-rolled at a finishing temperature of 900°C in the austenite region to a thickness of 3.0 mm, and subsequently water cooled to 650°C (specimen P), 500°C (specimen B) and below 100°C (specimen M) by water jet, and then they were cooled to room temperature by air. The temperature during cooling was evaluated by measuring surface temperature. Specimen P consisted of ferrite and pearlite, specimen B consisted of bainitic structures and specimen M consisted of fully martensitic structures. The hot-rolled sheets were cold-rolled to a thickness of 1.0 mm (reduction: 67%) by four-high cold rolling mill at a rolling speed of 15 m/min. The microstructures of hot-rolled and cold-rolled sheets are shown for each specimen in our previous article.8)
After cold-rolling, the cold-rolled sheets were cut into 1.0 (thickness) × 10 × 10 mm specimens. The specimens were heated to the target temperature at a rate of 0.5°C/s using an electric furnace, and then water-quenched to room temperature within 2 s after the removal of specimens from the furnace. Some specimens were isothermally held at 750°C for 1000 s. The reason for the slow heating rate is to complete the recrystallization of ferrite during annealing at ferrite single region. In addition, the Ac1 and Ac3 temperatures of the tested steel were approximately 670°C and 820°C, and the intercritical annealing temperature in the previous study was up until 730°C.8) During the cooling process, the austenite phase, which was present during intercritical annealing, transformed into martensite and/or bainite. Also in the case of specimen M, the fraction of austenite during intercritical annealing corresponds to that of martensite and/or bainite after the cooling process because recrystallization of ferrite was completed at 610°C8) and the microstructure did not include initial martensite.
The microstructures of LePera-etched specimens were observed using optical microscopy. LePera etchant (1% Na2S2O5 + 4% picric acid in ethanol) has been used to show the existence of martensite clearly.9) The fraction of martensite and/or bainite was estimated by the point-count method (ASTM E 562), and this method was also applied in the previous study.8) The fraction of martensite and/or bainite is the value estimated from 2475 counting points. In addition, aspect ratio of martensite and/or bainite was evaluated from the microstructures observed by optical microscopy. The estimated fraction and aspect ratio of martensite and/or bainite corresponds to that of austenite during intercritical annealing since the austenite transforms into martensite and/or bainite during the cooling process. The partitioning of C and Mn between ferrite and austenite was measured using field emission-scanning electron microscope/energy dispersive X-ray spectroscopy (FE-SEM/EDS) at an accelerating voltage of 15 kV and EDS line analysis was performed.
Figure 1 shows the typical microstructures of LePera-etched specimen heated to 700, 730 and 750°C. The white regions in Fig. 1 correspond to austenite (i.e., martensite and/or bainite). In the previous study,8) ferrite recrystallization was confirmed to be completed before intercritical annealing. Furthermore, it was also apparent that the regions of austenite in all the specimens increased with the annealing temperature. For the specimens heated to 700°C, cementite particles were still observed (Figs. 1(a)–(c)). In the case of the specimens heated to 730°C, austenite was connected and elongated along the rolling direction in specimens P and B, while it was distributed homogeneously in specimen M (Figs. 1(d)–(f)). However, it should be noted that austenite was connected and elongated along the rolling direction in all the specimens at 750°C (Figs. 1(g)–(i)). In addition, it is apparent that the fraction of austenite in specimen M is larger than that in specimen P at 730°C (Figs. 1(d) and (f)), while that in specimen M is smaller than that in specimen P at 750°C (Figs. 1(g) and (i)).
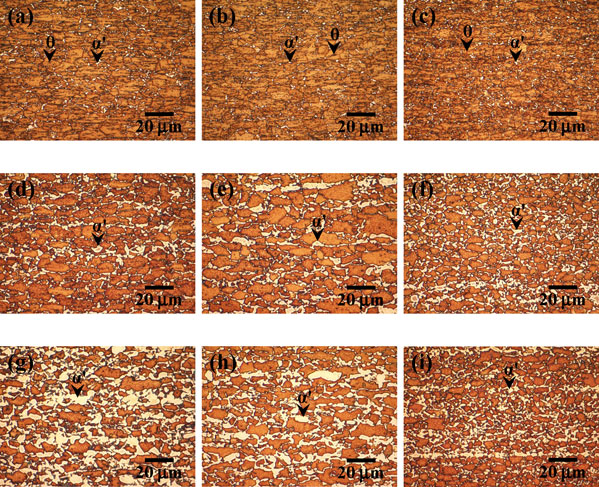
Typical microstructures of LePera-etched specimens (a) P, (b) B and (c) M heated to 700°C, specimens (d) P, (e) B and (f) M heated to 730°C, and specimens (g) P, (h) B and (i) M heated to 750°C (α′: martensite and/or bainite, θ: cementite particles).
Figure 2(a) shows the changes in the austenite fraction of each specimen annealed at various intercritical temperatures. At 700°C, the austenite fraction in all specimens was almost the same. The austenite fraction in specimen M was previously demonstrated to be larger than that in specimens P and B in the temperature range from 710 to 730°C.8) However, it should be noted that the austenite fraction was changed to the order of P > B > M by intercritical annealing above 740°C. Aspect ratio of austenite in each specimen heated to 730 and 750°C is shown in Fig. 2(b). The aspect ratio of austenite in specimens P and B annealed at 730°C was above 1.0, and this result suggests that austenite was connected and elongated along the rolling direction. In contrast, the aspect ratio in specimen M annealed at 730°C was approximately 1.0, and this result suggests that austenite was distributed homogeneously. At 750°C, austenite in all specimens was connected and elongated along the rolling direction because the aspect ratio of austenite was above 1.0.
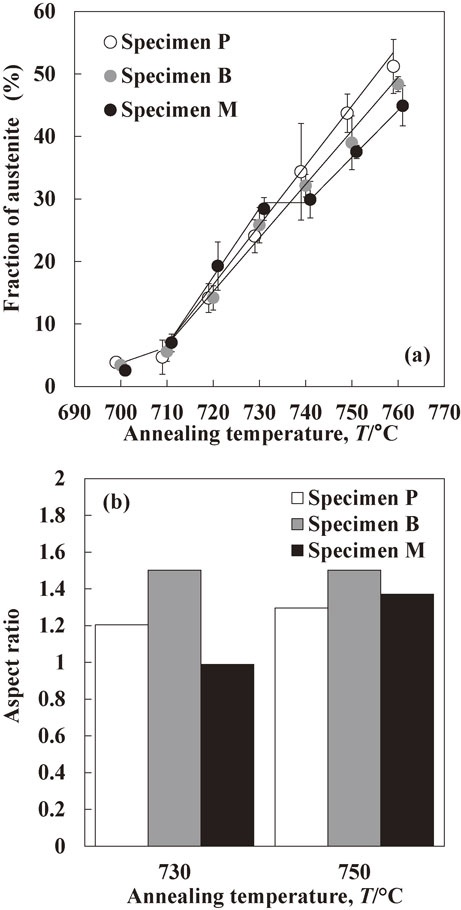
(a) Changes in the austenite fraction of specimens P, B and M as a function of annealing temperature (Data from 700 to 730°C was taken from a previous report.8)), (b) aspect ratio of austenite in specimens P, B and M heated to 730 and 750°C.
Figure 3(a)–(c) shows the line analysis results for C and Mn in each specimen heated to 750°C. For all the specimens, a partitioning of C between the ferrite and austenite (i.e., martensite and/or bainite) phases was confirmed, whereas the Mn distribution was homogeneous across both phases. Figure 3(d)–(f) shows the line analysis results for C and Mn in each specimen annealed at 750°C for 1000 s. A partitioning of Mn in the ferrite and austenite phases was confirmed in specimens P and B, whereas it was scarcely observed in specimen M.
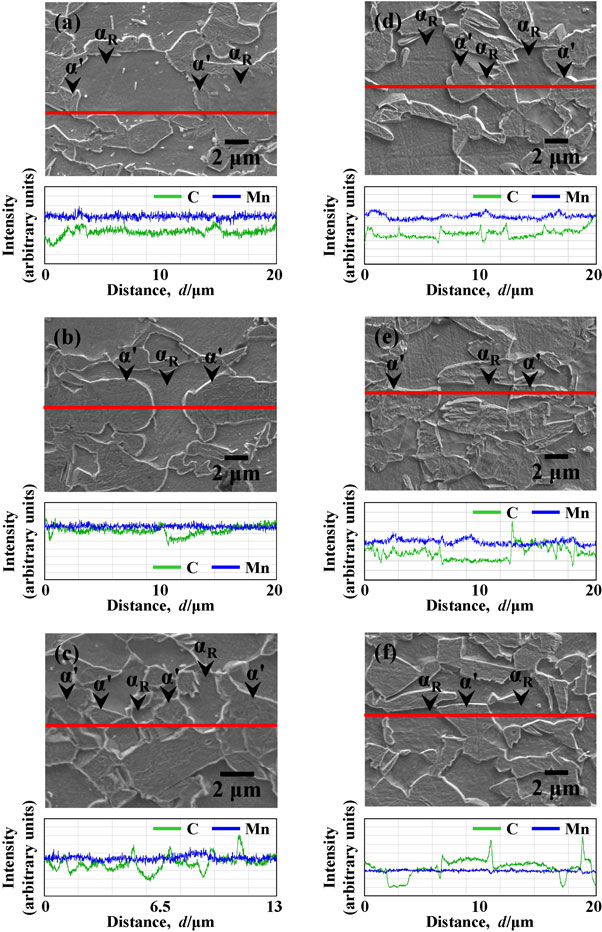
Line analysis for C and Mn in specimens (a) P, (b) B and (c) M heated to 750°C, and in specimens (d) P, (e) B and (f) M annealed at 750°C for 1000 s.
One noteworthy finding in the present study is that the austenite formation behavior in specimens P, B and M is quite different at lower (below 730°C) and higher (above 740°C) temperatures. We have reported that the austenite fraction in specimen M was larger than that in specimens P and B below 730°C.8) This is because the number of austenite nucleation sites during intercritical annealing in specimen M is larger than those in specimens P and B. We have revealed using transmission electron microscopy (TEM) and electron back scattering diffraction (EBSD) that austenite nucleation sites are mainly at high-angle grain boundaries such as those between recrystallized ferrite grains.1,2) As shown in Fig. 1, a major portion of austenite (i.e., martensite and/or bainite) was observed at recrystallized ferrite grain boundaries also in this study. In contrast, as shown in Fig. 2(a), the austenite fraction was in the order of P > B > M above 740°C. In particular, it should be noted that the austenite fraction in specimen M did not change significantly during heating from 730 to 740°C. It has been reported that the nucleation and growth of austenite in C–Mn steels proceeds in several stages, and the dissolution of cementite and/or pearlite occurs first.10) After the dissolution of cementite and/or pearlite, austenite growth with C diffusion in austenite occurs subsequently (paraequilibrium), and then austenite growth with Mn diffusion in ferrite occurs (local equilibrium). For all specimens heated to 750°C, a partitioning of C was observed in the ferrite and austenite phases, whereas Mn was distributed homogeneously across both phases (Fig. 3(a)–(c)). In the case of annealing at 750°C for 1000 s, a slight partitioning of Mn was observed in the ferrite and austenite phases of specimens P and B, whereas this was not confirmed in specimen M (Fig. 3(d)–(f)). These results suggest that the kinetics of austenite formation in specimen M is different from those in specimens P and B. It has been demonstrated that austenite growth during annealing proceeds under paraequilibrium when the heating rate is rapid, whereas the same proceeds under local equilibrium when the heating rate is slow.11) In addition, the recrystallization of ferrite was completed when the heating rate was slow, while non-recrystallized ferrite grains were observed when the heating rate was rapid.4) We have reported that the number of austenite nucleation sites increases with the fraction of non-recrystallized ferrite grains when the annealing temperature reaches the Ac1 temperature.4) Therefore, it is likely that austenite growth tends to proceed under local equilibrium when the number of austenite nucleation sites is small, whereas austenite growth tends to proceed under paraequilibrium when the number of austenite nucleation sites is large. At lower temperatures (below 730°C), the nucleation and growth of austenite may proceed under local equilibrium in specimens P and B, whereas it proceeds under paraequilibrium in specimen M. In addition, the austenite growth proceeds under local equilibrium in all specimens at higher temperatures (above 740°C). It has been reported that Mn diffuses only at ferrite/austenite interfaces when the fraction of austenite is low,1,12) and the EDS elemental line scan profiles obtained in the previous study12) are similar to those shown in Fig. 3. Moreover, we have revealed using TEM that austenite growth at the latter stage of intercritical annealing is controlled by the volume diffusion of substitutional elements such as Mn at the ferrite and austenite interfaces.1,2) It appears that the austenite fraction in specimen M did not change significantly during heating from 730 to 740°C because the kinetics of austenite formation transitioned from paraequilibrium to local equilibrium.
A comparison of specimens P and B revealed the austenite fraction in specimen P was larger than that in specimen B above 740°C (Fig. 2(a)). The growth rate of austenite into pearlite has been reported to be higher than that into proeutectoid ferrite.13) This finding indicates that as more local carbon-rich areas (e.g. pearlite colonies) increase, the austenite growth rate increases. Thus, the austenite fraction in specimen P was probably larger than that in specimen B because the initial microstructure of specimen P includes local carbon-rich areas such as pearlite colonies.
In the viewpoint of austenite distribution, austenite was connected and elongated along the rolling direction in specimens P and B, while it was distributed homogeneously in specimen M at 730°C. This result agrees with our previous study.8) In contrast, austenite was connected and elongated along the rolling direction in all the specimens at 750°C. For 0.1C–2.0Mn steel, we have demonstrated that the rate-controlling mechanism of grain growth during intercritical annealing is volume diffusion of Mn in the austenite phase.2) Thus, austenite nucleation sites are mainly at high-angle grain boundaries such as those between recrystallized ferrite grains, but austenite may not necessarily grow along the recrystallized ferrite grain boundaries. For specimen M, the austenite distribution was homogeneous at the early stage of annealing (730°C), but it is likely that austenite was connected and elongated along the rolling direction because austenite grew not only along the recrystallized ferrite grain boundaries but also within through the recrystallized ferrite grains at the latter stage of annealing (750°C).
Figure 4 schematically illustrates the microstructural evolution and distribution of C and Mn during heating from 730 to 750°C for each specimen. Relationship between microstructural evolution and distribution of C and Mn is clearly shown in Fig. 4.
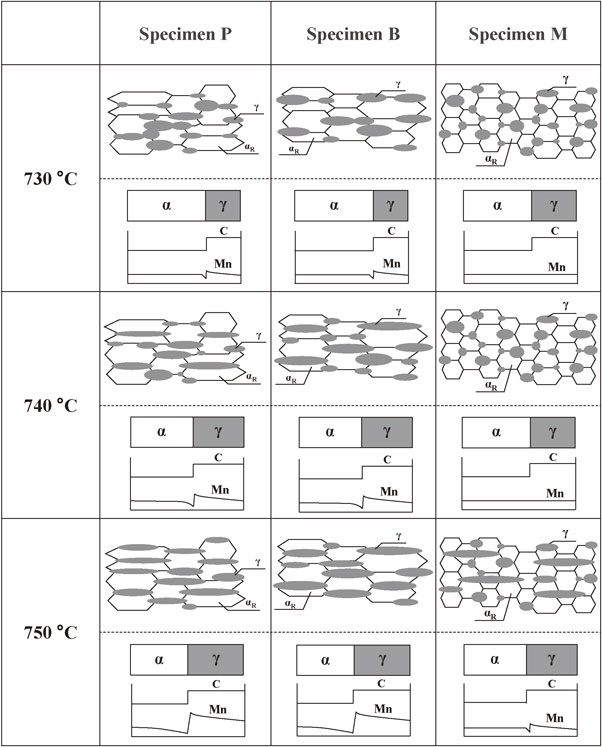
Schematics showing microstructural evolution and distribution of C and Mn during heating from 730 to 750°C for each specimen (αR: recrystallized ferrite, γ: austenite).
The effect of initial microstructures on austenite formation behavior in low-carbon steel during intercritical annealing was investigated. The austenite fraction in specimen M was larger than that in specimens P and B below 730°C, whereas the order of specimens was P > B > M above 740°C. Below 730°C, austenite connected along the rolling direction was observed in specimens P and B, while the distribution of austenite in specimen M was uniform. In contrast, austenite was connected and elongated along the rolling direction in all the specimens above 740°C. The nucleation and growth of austenite can proceed under local equilibrium in specimens P and B, whereas it can proceed under paraequilibrium in specimen M below 730°C. In addition, austenite growth can progress under local equilibrium in all specimens above 740°C.
This work was supported by a research grant from the Hitachi Metals · Materials Science Foundation, Japan.