2019 Volume 60 Issue 1 Pages 111-120
2019 Volume 60 Issue 1 Pages 111-120
The thermodynamic properties for Nd2(MoO4)3 were investigated. Nd2(MoO4)3 is one of the end member of the yellow phases which are known as hygroscopic harmful phases in the nuclear fuel waste glasses. The standard molar entropy, 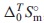 , at 298.15 K of Nd2(MoO4)3 was determined by measuring its isobaric heat capacities,
, at 298.15 K of Nd2(MoO4)3 was determined by measuring its isobaric heat capacities, 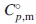 , from 2 K via the fitting functions including the Debye-Einstein formula and electronic- as well as magnetic terms. The Neel temperature, TN, estimated by extrapolating the magnetic-term in the fitting function. Its standard Gibbs energy of formation,
, from 2 K via the fitting functions including the Debye-Einstein formula and electronic- as well as magnetic terms. The Neel temperature, TN, estimated by extrapolating the magnetic-term in the fitting function. Its standard Gibbs energy of formation, 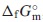 , was determined by combining
, was determined by combining 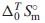 datum with the standard enthalpy of formation,
datum with the standard enthalpy of formation, 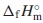 , which were estimated from ones for Ce2(MoO4)3 and Sm2(MoO4)3. The unknown standard Gibbs energy of solution,
, which were estimated from ones for Ce2(MoO4)3 and Sm2(MoO4)3. The unknown standard Gibbs energy of solution, 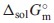 , at 298.15 K of Nd2(MoO4)3 was predicted from the reference data for MoO42−(aq) and Nd3+(aq). The obtained thermodynamic values are as follows:
, at 298.15 K of Nd2(MoO4)3 was predicted from the reference data for MoO42−(aq) and Nd3+(aq). The obtained thermodynamic values are as follows:
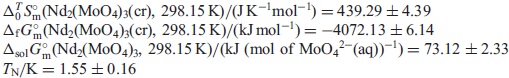
The data obtained in the present work are expected to be useful for geochemical simulations of the diffusion of radioactive elements through underground water.
This Paper was Originally Published in Japanese in J. Japan Inst. Met. Mater. 81 (2017) 485–493. To more precisely express the physical meaning of the functions for fitting the heat capacity, some their coefficients and thermodynamic values for Nd2(MoO4)3 were revised. The thermodynamic values for the relevant molybdates were revised based on the updated standard entropy at 298.15 K of molybdenum as the standard states. See Appendix describing the revised details.