2019 Volume 60 Issue 1 Pages 19-24
2019 Volume 60 Issue 1 Pages 19-24
The NaCl–Na2CO3 salt mixture is proposed as a water-soluble core material for aluminum die-casting processes. The mechanical properties and microstructures of NaCl–Na2CO3 samples prepared by gravity casting were investigated. The salt mixtures had superior properties compared to those of the pure salts. Plastic deformation occurred during compression tests at high temperatures because the eutectic region changed from lamellar to granular. Salt mixtures with NaCl primary phase and eutectic regions were found to be the most suitable core material.
Die-cast aluminum components are widely used in the automotive industry, because they have high productivity as well as excellent mechanical properties. In order to improve the performance of components and to save cost, complex shapes and parts with internal cavities must be cast, which is achieved through the use of cores. Metal or sand cores are commonly used for aluminum die-casting processes: however, water-soluble salts are more attractive as core materials as they can be easily removed from cavities that are not accessible to mechanical cleaning. Salt cores formed by high-pressure compaction of NaCl powder are the most common type used: however, they lack strength, so can only be used for gravity or low-pressure casting.1)
Many high-strength salt cores have therefore been proposed for use in high-pressure die-casting. For example, Yaokawa et al.2) proposed composites of salts and ceramics, and Jelínek and Adámková3) proposed the NaCl or KCl cores by high-pressure squeezing using alkali silicate. Our group has previously presented high-strength salt-mixture cores of the KCl–NaCl–K2CO3–Na2CO3 system made by gravity casting.4) The developed core materials casted by die-casting machine showed high dimensional accuracy, smooth surface and higher strength than those of gravity casting,5) and we successfully cast ADC12 alloy closed deck type cylinder blocks by high-pressure die-casing process.5,6) We have further developed this salt mixture to the KBr–NaBr–K2CO3–Na2CO3 system, which showed better removability due to higher solubility in water.7) However, the mechanical properties of these salt mixtures were only tested at room temperature. High-temperature mechanical properties of salt mixtures are a useful knowledge of casting design consideration because the salt cores are exposed to high-speed aluminum alloy melt during die-casting process. But high-temperature mechanical properties of our developed salt cores at high temperature are still unknown.
The high-temperature mechanical properties of single and polycrystalline NaCl have been investigated by many researchers.8–15) Single-crystal NaCl shows plastic deformation even at room temperature,12) whereas polycrystalline NaCl is brittle until heated to above half its melting point, whereupon it also begins to show plastic behavior. The mechanical strength of polycrystalline NaCl reaches a maximum at around 200–350°C.14,15) However, the mechanical properties of salt mixtures at elevated temperatures have not yet been documented. In this study, we investigate the high-temperature mechanical properties of cast salt-mixture cores of the NaCl–Na2CO3 system at elevated temperatures were investigated.
Gravity-cast salt-mixture samples were prepared by melting high-purity NaCl_(99.5%) and Na2CO3_(99.5%) in an electric furnace in air. The melting temperature was 10°C higher than the liquidus temperature for each composition. The molten salts were then poured into a steel mold in which large risers were set to avoid solidification shrinkage, as shown in Fig. 1(a). The mold was preheated to 100°C. Samples were ejected from the mold 60 s after pouring and cooled to room temperature. A photograph showing the appearance of the as-cast salt mixture is given in Fig. 1(b). Seven cylindrical samples (diameter 5 mm) were obtained.

Appearance of (a) permanent die and (b) cast salt mixture.
The sample names and chemical compositions are listed in Table 1, and calculated phase diagram of the NaCl–Na2CO3 system16) is shown in Fig. 2 along with the liquidus temperatures for each composition. The primary phase of sample 2CO3 is of NaCl, that of 6CO3, 8CO3 and 9CO3 are of Na2CO3: ECO3 corresponds to the eutectic composition.


Calculated phase diagram of NaCl–Na2CO3 with liquidus temperatures of specimens.
Compression tests were conducted on the gravity-cast cylinders (5 mm diameter, 12 mm high) at temperatures between room temperature and 250°C in air at a cross-head speed of 5 mm/s. 250°C is upper limit of our instrument. Typical sample appearance after compression test is shown in Fig. 3. Many of samples were fractured at the middle of sample. Micro Vickers hardness was measured on the as-cast sample surface under a load of 4.9 N for 30 s at room temperature. The hardness was calculated as an average of five measurement points.

Typical appearance of sample after compressive test.
The microstructure of the specimens was examined by scanning electron microscopy (SEM). As-cast samples and those after compression to a strain of 0.15 or 0.2 at 250°C were analyzed. For observation, the specimen surface was polished carefully with grade #4000 SiC, and then the polished surface was etched with methanol (99.8%).
True stress–true strain (σ–ε) curves for compression tests between room temperature and 250°C are shown in Fig. 4. Although the salt mixtures showed brittle fracture in the elastic region at room temperature, they showed plastic deformation at elevated temperatures. Sample 2CO3 with the NaCl primary phase, showed improved ductility above 50°C, where as the other samples only showed improvement above 150°C. Most of the σ–ε curves showed failure near the maximum stress, with the exception of those for samples 2CO3 above 200°C, where the maximum strain corresponded to decreasing flow stress, and that for sample ECO3 at 250°C, which was almost constant after yielding. Figure 5 summarizes the temperature dependence of the maximum stress σmax and fracture strain εf. The largest σmax for the 2CO3 and ECO3 samples was measured at 50°C, while that for samples 6CO3, 8CO3, and 9CO3 was measured above 150°C with increasing εf. σmax and εf for pure Na2CO3 were both very small.

True stress–true strain (σ–ε) curves at various temperatures.
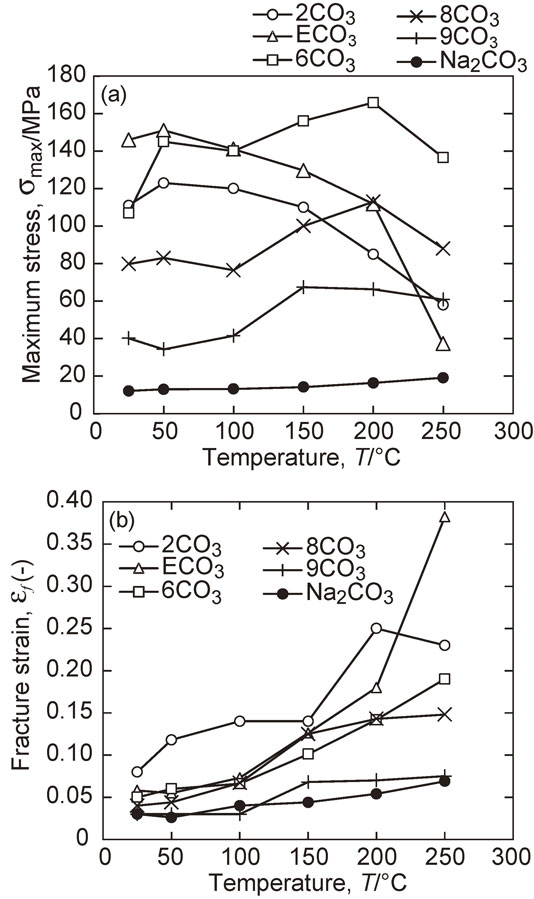
Temperature dependence of the maximum stress and fracture strain.
Figure 6 shows the Vickers hardness of the primary NaCl, primary Na2CO3, and eutectic region of each sample at room temperature. The primary NaCl phase was very soft compared to the primary Na2CO3 phase, and the hardness of the eutectic region took an intermediate vale.

Micro-vickers hardness at room temperature.
As the compression tests were carried out at 250°C, to isolate the effect of compression on the microstructure, 6CO3 sample were annealed at 250°C for 1 h to identify the effect of the heat treatment. Microstructures of the as-cast and annealed 6CO3 samples are shown in Fig. 7. There were no significant changes after heat treatment. Thus, any changes to the microstructure after compression tests were produced by the compression as opposed to heat treatment.

Microstructure of sample 6CO3: (a), (b) as-cast condition and (c), (d) after annealing at 250°C for 1 h.
Figure 8 shows the microstructure of the as-cast and compressed (strain of 0.2 at 250°C) 2CO3 samples. The as-cast sample consist of white regions of primary NaCl and a lamellar eutectic region, as shown in Figs. 8(a) and 8(b). A large section of one of the primary NaCl phases detached during etching, leaving large hole. After the compression test, the primary NaCl region was slightly distorted. Moreover, the lamellar structure in the eutectic region had dramatically changed to a granular structure as shown in Fig. 8(d).

Microstructure of sample 2CO3: (a), (b) as-cast condition and (c), (d) after compression test at 250°C.
Figure 9 shows the microstructure of the as-cast and compressed (strain of 0.15 at 250°C) ECO3 samples. Although a small amount of primary Na2CO3 can be observed, most of the as-cast sample consists of a fine lamellar eutectic structure, with distinct edges of eutectic colonies, as shown in Fig. 9(a). After the compression test, part of the lamellar structure coarsened and changed to a granular structure, as shown Fig. 9(b).

Microstructure of ECO3: (a) as-cast condition and (b) after compression test at 250°C.
Microstructures of the as-cast and compressed (strain of 0.15 at 250°C) 8CO3 samples are shown in Fig. 10. Large grains of primary Na2CO3 phase were observed, surrounded by the lamellar eutectic structure. There was no change in the primary Na2CO3 phase after compression test, while the lamellar structure in eutectic region changed to a granular structure, as shown Fig. 10(b), similarly to Figs. 8(d) and 9(b). Therefore, microstructural changes in the eutectic region are thought to account for the plasticity showed by the salt mixtures at elevated temperatures.

Microstructure of sample 8CO3: (a) as-cast condition and (b) after compression test at 250°C.
The maximum strength σmax and fracture strain εf of the salt mixtures studied in the present work are several times larger than those of the constituent parts. The mechanical properties of pure NaCl have been studies previously:14) polycrystalline samples show a brittle-to-ductile transition at around half the melting temperature, with plastic deformation observed above 156°C: yielding strength is less than 20 MPa at 156°C. The mechanical properties of pure Na2CO3 could not find in the literature: however, according to the results presented in Fig. 4(f), plastic deformation does occur, although the strain to fracture is less than 0.08. The yielding strength of pure Na2CO3 is between 15 to 20 MPa. From these facts, this improvement of mechanical properties of salt mixture is likely to be caused by the eutectic structure, because 9CO3 samples, which had only a small volume of eutectic, showed poor ductility. Furthermore, the microstructure of the eutectic region changed from a lamellar to a granular structure after compression test at 250°C, as shown in Figs. 8–10.
The largest maximum stress (σmax) at room temperature was that of ECO3 sample. The fine lamellar structure is considered to be the cause of the high strength. However, σmax decreased and εf increased with increasing temperature. The mechanism of plastic deformation in the eutectic region is not clear from the present work, although it is hypothesized that dynamic recrystallization occurred which induced a microstructure change, similar to that in other alloys.17)
Required mechanical strength of salt cores depends on casting design. For example, Lagler simulated stress in a salt core during high-pressure die-casting by numerical simulation.18) He suggested that the intensification pressure should be after the filling process completed. The maximum stress in salt core during filling process was about 15 MPa at the ingate velocity 120 m/s for his simulation. From this result, the present salt-mixtures have enough mechanical strength as salt core except pure Na2CO3. Optimum composition as salt core would be selected by a factor beside strength. At high temperature, σmax is higher for samples 2CO3 and 6CO3 than for ECO3 samples. Of these, 6CO3, which includes primary Na2CO3 has greater than 2CO3 which includes primary NaCl. However, the fracture strain of 2CO3 is larger than 6CO3 and also 2CO3 has the better ductility at low temperatures. Furthermore, impact strength and thermal shock resistance of 2CO3 better than 6CO3, which will be reported in elsewhere. The salt mixture which includes primary NaCl is the more suitable core material in order to avoid the fracture of core during process.
NaCl–Na2CO3 salt mixtures were prepared by gravity casting. Their mechanical properties of were investigated by compression tests, and the following results were obtained: