2019 Volume 60 Issue 10 Pages 2174-2178
2019 Volume 60 Issue 10 Pages 2174-2178
The effects of surface V species on the hydrogen permeability through V membrane without Pd-catalyst overlayer are investigated by the hydrogen permeation test at 773 K and the XPS measurement. The steady-state hydrogen permeation flux through Pd-coating free V membrane after the redox treatment is more than twice that of the non-treated membrane. The hydrogen flux after 50 hours, however, are almost the same regardless of the redox treatment since the redox-treated membrane is deactivated during the hydrogen permeation test. The fraction of V0+/V2p3/2 on the surface of membrane just after the redox treatment is approximately 2%, and the amount of V3+ is larger than V4+. This fine balance of the V species composition on the surface is found to induce the high hydrogen permeability. The composition of V species on the surface of the redox-treated membrane changes to V3+ < V4+ by oxidation of V3+ to V4+ during the hydrogen permeation test for a long time. The loss of the fine balance in the composition of V species is considered to be the cause of the degradation of hydrogen permeability.

Fig. 2 Change in the hydrogen permeation flux through Pd-coating free V membrane with respect to time for 3 hours at 773 K under the hydrogen pressure difference of 0.1 MPa.
The group 5 metals on the periodic table, i.e. V, Nb and Ta, are well known to exhibit the highest hydrogen permeability among metals.1,2) Taking advantage of the excellent hydrogen permeability, the group 5 metals-based hydrogen permeable alloy membranes have been developed as a substitute for Pd or its alloy membranes for hydrogen separation and purification from the mixture gas.3–13) The practical hydrogen flux, however, cannot be unfortunately obtained by using a simple membrane which is constructed with the group 5 metals or its alloys. Since the group 5 metals are oxidized easily, an oxide layer is formed uniformly on the surface of membrane. The oxide layer with poor catalytic activity for hydrogen have been understood to inhibit hydrogen permeation reaction.14) In the research field of non-Pd-based hydrogen permeable metal membrane, the hydrogen permeability has been evaluated using the composite membrane with a Pd-catalyst overlayer in order to avoid the problem of surface oxide layer.15) The composite membrane, however, have disadvantage of poor durability since the Pd-catalyst overlayer deteriorates at high temperature or during a longtime hydrogen permeation reaction. The valid solution to improve the durability have not yet been reported.
It has been recently reported that the hydrogen permeation reaction through the pure V metal membrane without Pd-catalyst overlayer can be observed at above 773 K.16,17) The results, described in these papers, that the redox treatment improves the hydrogen permeability through Pd-coated free V membranes is very interesting. The V2O3 formed on the surface of the redox-treated membrane is explained to contribute to the hydrogen permeation reaction based on the results of X-ray diffraction, XRD, measurement. The hydrogen permeability through the redox-treated membrane, however, has been confirmed to be very unstable. The V species other than V2O3 present on the outermost surface, which cannot be detected by XRD measurement, might affect hydrogen permeability. The conditions of the redox treatment and the hydrogen permeation reaction must be strictly controlled in order to obtain high hydrogen permeability. In this paper, in order to clear the effect of the surface oxidation-state on the hydrogen permeability, the composition of V species on the surface of Pd-coating free V membrane before and after the hydrogen permeation test is investigated by the X-ray photoelectron spectroscopy, XPS. While the previous paper described about the crystal structure based on the XRD measurement, we will discuss for first time the effect of redox treatment on the V species for the surface of Pd-coating free V membrane.
The samples for hydrogen permeation test and XPS measurement are prepared from high purity V rolling material with the thickness of 200 µm and purity of 99.9 mass% provided by Taiyo Koko Co., Ltd. The V membrane sample with the diameter of 12 mm and the thickness of 200 µm is cut out using a die-sinking electric discharge machining, and ultrasonic cleaned in acetone and pure water for 5 minutes, respectively. The membrane sample is annealed at 1273 K in a vacuum of 5 × 10−3 Pa or less for 15 minutes in order to remove the processing strain as the final process.
2.2 Hydrogen permeation testFigure 1 shows the schematic illustrations of the test apparatus to evaluate the hydrogen permeability. Figure 1(a) is a gas-piping diagram, and Fig. 1(b) is a cross-sectional view of membrane sample holder. The membrane sample is sandwiched between SUS316-gaskets and fixed using a Swagelok VCR fittings as the sample holder. The sample holder is heated to 773 K using a tubular electric furnace while evacuating the inside of the gas-piping. Then the high-purity hydrogen gas (>99.999%, Air Liquid Japan Ltd.) is introduced into the sample holder. The hydrogen pressure in the inlet and outlet side of the membrane sample is adjusted to 0.2 MPa and 0.1 MPa, respectively. The hydrogen permeation flux, Jd/mol H m−1s−1, normalized with a unit thickness, a unit area and a unit time through membrane sample is estimated from the gas flow rate, Q/cc min−1, in outlet-side measured using a digital mass flow meter. The details of the test and analysis method for hydrogen permeability have been explained in the previous papers.18)
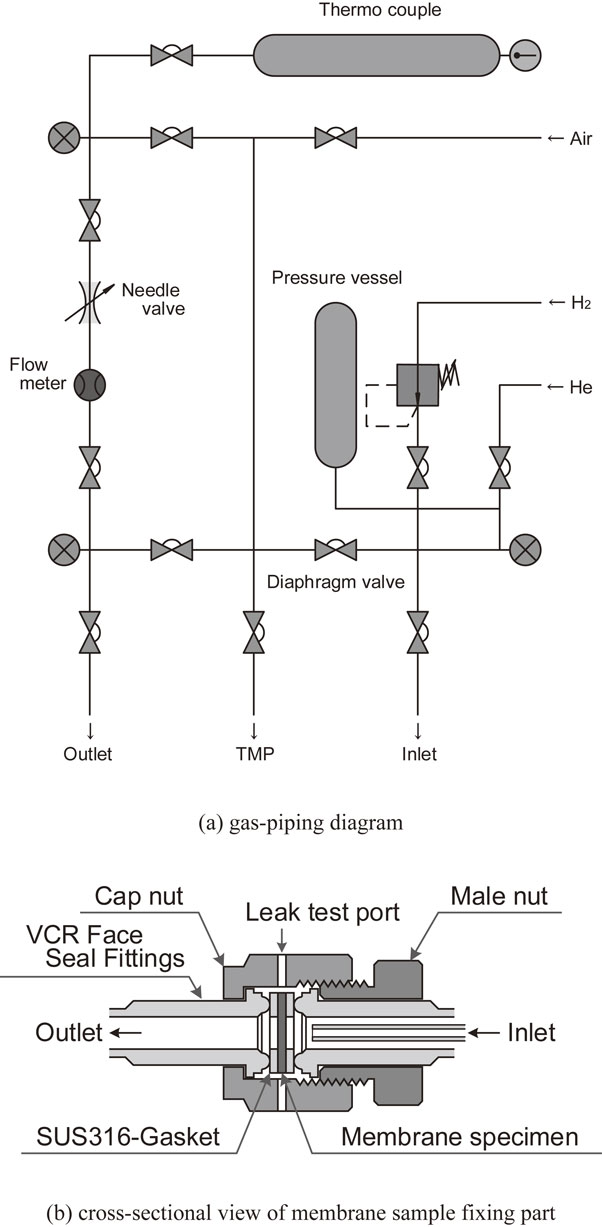
Schematic illustrations of the apparatus for hydrogen permeation test; (a) gas-piping diagram and (b) cross-sectional view of membrane sample holder.
The changes in the hydrogen permeability through Pd-coating free V membrane with and without the redox treatment are compared in this experiment. The redox treatment of the membrane surface is performed in the following steps as a pretreatment for evaluating the hydrogen permeability. In the oxidation step, after heating the membrane sample to 773 K, air is introduced into the sample holder and left for 1 minute. In the reduction step, after evacuating the inside of the sample holder for 5 minutes, hydrogen gas is introduced to 0.2 MPa and left for 5 minutes. The reduction step is repeated twice after one oxidation step as a redox treatment process just before measuring the hydrogen permeation flux.
2.3 XPS measurementThe valence of V on the membrane surface is analyzed by XPS (XPS, PHI 5450LC, ULVAC-PHI, Inc.). The XPS spectrum is recorded using a non-monochromatic Mg-Kα X-ray source of 400 W under an operating pressure of 10−7 Pa. The membrane sample is fixed to the sample holder with a gold mesh as a standard substance. The binding energy is corrected with reference to the 4f7/2 peak of Au at 84.0 eV.19) The composition of V species is determined by the peak separation analysis for V 2p3/2 from 510 eV to 520 eV.
Figure 2 shows the change in the hydrogen permeation flux of Pd-coating free V membrane with respect to time for 3 hours at 773 K under the hydrogen pressure difference of 0.1 MPa. The hydrogen flux of membrane sample without redox treatment indicated with the broken line gradually increases with the hydrogen pressure difference and reaches a steady-state flux of 29 mol H m−1s−1 after about 1.3 hours. In case of membrane sample with redox treatment, indicated with solid line, the hydrogen flux reaches a steady-state flux of 67 mol H m−1s−1 after 0.8 hours. The hydrogen permeability through the redox-treated membrane at the steady-state is more than twice that of the non-treated membrane. The redox treatment for Pd-coating free V membrane is found to reduce the time to reach the steady-state and to improve the initial performance of the hydrogen permeability.
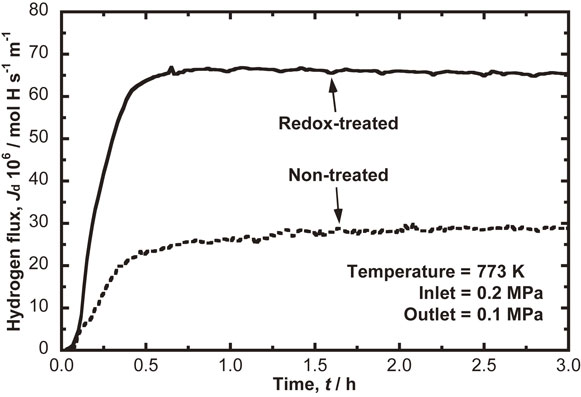
Change in the hydrogen permeation flux through Pd-coating free V membrane with respect to time for 3 hours at 773 K under the hydrogen pressure difference of 0.1 MPa.
The change in the hydrogen flux of the membrane sample used in Fig. 2 with respect to time extended to 150 hours is shown in Fig. 3. Although the redox-treated membrane shows excellent initial performance compared to the non-treated membrane, the hydrogen flux after 50 hours are almost the same regardless of the redox treatment. The redox-treated membrane is found to be deactivated during the hydrogen permeation reaction for about 50 hours at 773 K. Incidentally, the hydrogen flux change that repeats increase and decrease in a 24-hour cycle is observed in the result of the non-treated membrane. This phenomenon is an inherent problem of the apparatus shown in Fig. 1 and caused by the fluctuation of hydrogen pressure depending on the environment in outlet-side. The properties of the membrane, therefore, are not the cause of the periodically repeated phenomenon.

Change in the hydrogen permeation flux through Pd-coating free V membrane with respect to time for 150 hours at 773 K under the hydrogen pressure difference of 0.1 MPa.
Figure 4(a) and (b) show the V2p3/2-XPS spectra of Pd-coating free V membrane without and with redox treatment, respectively. The measured XPS spectrum indicated with broken line can be separated into the spectra of V0+, V2+, V3+, V4+ and V5+ indicated with solid lines. The V species of 0 to 5 valences are observed on the surface of V membrane regardless of the redox treatment. The fraction of V0+/V2p3/2 on the membrane surface decreases from 10% to 2% by the redox treatment. Although it is not shown in the figure, the XPS spectrum measured after the oxidation step in the redox treatment is constructed with only V5+. V0+ hardly reappears even if V5+ is reduced with H2 gas as shown in Fig. 4(b). Since the initial performance is improved by the redox treatment as shown in Fig. 3, the decreasing V0+ can be understood to contribute to hydrogen permeation reaction through Pd-coating free V membrane.
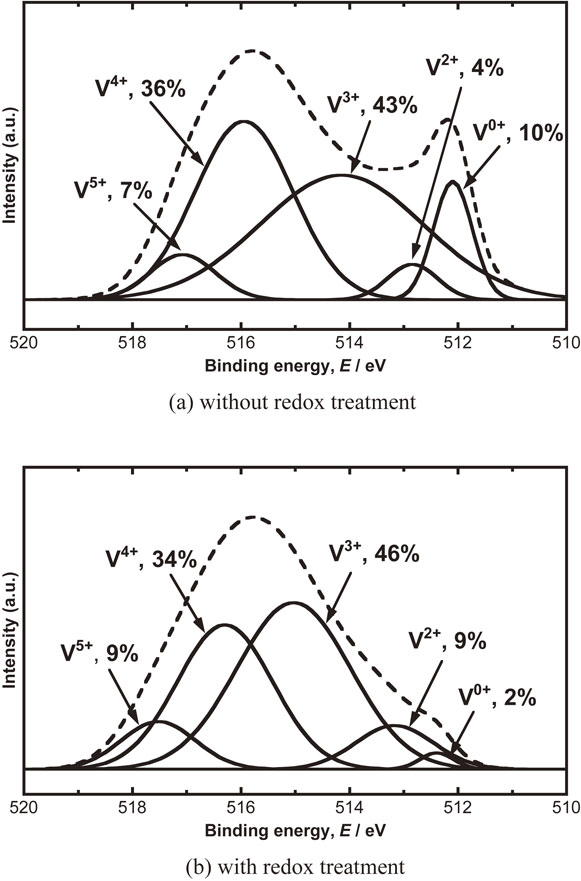
V2p3/2-XPS spectra of Pd-coting free V membrane before hydrogen permeation test; (a) without redox treatment and (b) with redox treatment.
Figure 5 shows the V2p3/2-XPS spectra of Pd-coating free V membrane after hydrogen permeation test at 773 K shown in Fig. 3. Figure 5(a) to (d) are spectra of inlet and outlet side for membrane without and with redox treatment, respectively. The composition of the V species on the inlet-side surface is similar between membrane with and without redox treatment. Focusing on the inlet-side shown in Fig. 5(a) and (c) compared with Fig. 4(a) and (b), the fraction of V0+/V2p3/2 on the surface of non-treated membrane decreases from 10% to 2% during the hydrogen permeation test. The redox-treated membrane keeps the fraction of V0+/V2p3/2 at about 2%. The fraction of V3+/V2p3/2 decreases from 43% to 26% (non-treated) and from 46% to 31% (redox-treated). In contrast, the fraction of V4+/V2p3/2 increases from 36% to 65% (non-treated) and from 34% to 60% (redox-treated). In case of the non-treated membrane, the time to reach the steady-state flux is longer because the V0+ needs to be oxidized in order to permeate smoothly the hydrogen. Since V3+ is also oxidized to V4+ during the oxidation of V0+ and the composition of V species changes to V3+ < V4+, the initial steady-state flux is understood to become lower as shown in Fig. 2. The initial composition of V0+ on the surface of redox-treated membrane is approximately 2% and the amount of V3+ is larger than V4+.
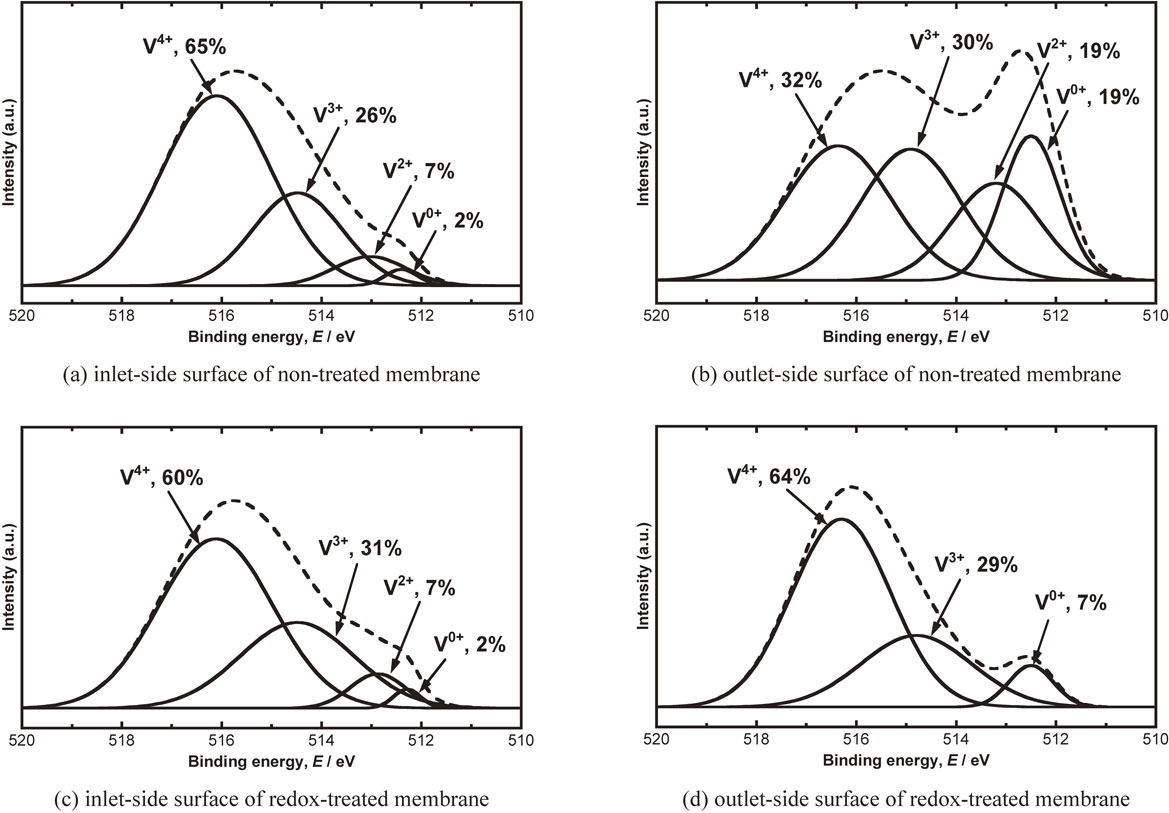
V2p3/2-XPS spectra of Pd-coating free V membrane after hydrogen permeation test at 773 K under the hydrogen pressure difference of 0.1 MPa; (a) non-treated membrane on inlet-side surface, (b) non-treated membrane on outlet-side surface, (c) redox-treated membrane on inlet-side surface and (d) redox-treated membrane on outlet-side surface.
These results are consistent with other papers explaining that the V2O3 layer promotes the hydrogen permeation reaction based on the XRD results.16,17) Although Fuerst et al. have shown the XPS results of various V surface as supporting information, the relationship between the changes of V species composition and the hydrogen permeability have not been discussed in detail. The fine balance of the V species composition on the outermost surface obtained by the redox treatment for the Pd-coating free V membrane is found to induce high initial hydrogen permeability in this paper. The hydrogen permeability through the redox-treated membrane, however, decrease to comparable level with the non-treated membrane because the composition of V species changes to V3+ < V4+ by oxidation of V3+ to V4+ during the hydrogen permeation test above 50 hours as shown in Fig. 3. It has been reported that V2O3 is formed by the reduction of V2O5 with H2 gas.20) Also in this study, a V3+-rich surface is obtained by the redox treatment as shown in Fig. 4(b). Referring to the Ellingham diagram, however, the stable phase under the test condition is VO2 because PH2/PH2O in the 99.999% H2 gas is around 105. Because V4+ becomes inevitably dominant species during the hydrogen permeation test, it is necessary to consider a method for stabilizing V3+ in order to obtain high-hydrogen permeability for a long time.
Focusing on the outlet-side shown in Fig. 5(b) and (d) compared with Fig. 4(a) and (b), the fraction of V0+/V2p3/2 increases after hydrogen permeation test regardless of the redox treatment. The hydrogen atoms diffusing in the membrane reduce the surface oxide resulting in an increase of V0+ on the outlet side. The presence of V0+ is considered to prevent the hydrogen permeation reaction as described above. The reduction by purified hydrogen at the outlet side is suggested to be also one of the causes of reducing the hydrogen flux through Pd-coating free V membrane.
The effects of the redox treatment on the hydrogen permeability through V membrane without Pd-catalyst overlayer have been investigated by the hydrogen permeation test and the XPS measurement. The redox treatment decreases the fraction of V0+/V2p3/2 on the surface and improves the initial hydrogen permeability through Pd-coating free V membrane. The hydrogen permeability through the redox-treated membrane decrease to comparable level with the non-treated membrane because the composition of V species changes to V3+ < V4+ by oxidation of V3+ to V4+ during the hydrogen permeation reaction for a long time. The Pd-coating free V membrane, just after the redox treatment, is found to exhibit high hydrogen permeability due to the fine balance of the V species composition on the surface.
The authors would like to express sincere thanks to Dr. Hideo Yoshinaga of Taiyo Koko Co., Ltd. for supporting us and preparing the samples in this study. This research was supported in part by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS) and CREST project (JPMJCR1443) from Japan Science and Technology Agency (JST).