2019 Volume 60 Issue 11 Pages 2267-2276
2019 Volume 60 Issue 11 Pages 2267-2276
Five Ir–Rh–Ru–W–Mo alloys selected based on alloy design with valence electron concentration (VEC) were examined for their formation of single, dual, and triple phases of bcc, fcc, and hcp structures. These structures were predicted with Thermo-Calc 2019a and the TCHEA3 database on a cross-sectional phase diagram along a composition line: Ir0.415254(100−2x)Rh0.415254(100−2x)Ru0.169492(100−2x)WxMox (x: 0–50 at%). At T = 2100 K, four types of phases were predicted: (1) a single bcc, fcc, and hcp phase, respectively, at x = 35 (Alloy A, VEC = 6.849), 15 (Alloy C, VEC = 7.981), and 5 (Alloy E, VEC = 8.574); (2) a mixture of bcc+hcp and hcp+fcc at x = 24 (Alloy B, VEC = 7.472) and 8 (Alloy D, VEC = 8.378), respectively; (3) a triple mixture of bcc+hcp+fcc; and (4) a mixture of bcc+fcc in Alloys A–E at low temperature. Experiments at 2100 K revealed that Alloys C–E tended to exhibit better reproducibility and that Alloy E can be regarded as a new refractory high-entropy alloy (HEA) with fcc structure. Alloy C annealed at T = 1273 K for 200 h maintained a single-hcp structure. The non-appearance of thermodynamically stable phases at low temperature in the Ir–Rh–Ru–W–Mo system was analogically explained as slow diffusion. The VEC analysis for HEAs with hcp structures was extended by including the range of 7.5 ≤ VEC ≤ 8.4 for alloys consisting of 4d and 5d transition metals annealed near their solidus temperature. The Ir–Rh–Ru–W–Mo system was significant in providing all possible simple solid solutions of bcc, hcp, and fcc phases.

Cross-sectional phase diagram predicted with Thermo-Calc 2019a and with the TCHEA3 database (broken blue and solid red lines) and experimental data (orange) by annealing samples. Red broken arrows roughly indicate the experimentally confirmed extension of the area of the HCP_A3 phase.
It goes without saying that high-entropy alloys (HEAs) have developed into the most attractive metallic materials since their first reports in 2004.1,2) The developmental progress of HEAs has been accompanied by the expansion of their definitions in terms of alloy composition and relevant quantities. Initially, HEAs were defined1) as alloys with exact equiatomicity and with five or more constituent elements, which corresponds to the description with the configuration entropy normalized by the gas constant (Sconfig/R) satisfying Sconfig/R ≥ 1.61.3) Here, Sconfig is given by eq. (1) with a fraction of the i-th elements (pi) in the alloy with N elements and is simply expressed as Sconfig = ln N in case of exact equiatomic alloy.
| \begin{equation} S_{\text{config}}/R = - \sum_{\text{i} = 1}^{\text{N}}p_{\text{i}} \ln p_{\text{i}} \end{equation} | (1) |
As well as changes of the definition of HEAs in terms of compositions, the structural types of HEAs have also been changed gradually. Specifically, the definition had long been limited to simple crystallographic structures, in particular, solid solutions of bcc, fcc, and their mixtures.1) Subsequently, HEAs with hcp structures have been found in the past several years. For instance, the constituent elements and/or production methods of HEAs with hcp structure reported to date consist of heavy lanthanide elements with6) and without6,7) Y, light-weight elements by mechanical alloying and subsequent transformation,8) 3d transition metals by applying high pressure,9) and 4d and 5d transition metals by chemical reaction.10) Following these reports, the authors have recently succeeded in fabricating HEAs with hcp structure for alloys from 4d and 5d elements in Ir26Mo20Rh22.5Ru20W11.5 and Ir25.5Mo20Rh20Ru25W9.5 alloys11) by conventional arc melting and subsequent annealing. A unique feature of these Ir–Mo–Rh–Ru–W HEAs11) is that the hcp structure of the alloys is controlled by valence electron concentration (VEC)12) ∼7.8. Specifically, the alloy design is supported by a concept of structural stability evaluated according to the enthalpy by Miedema’s model13,14) as a function of VEC. Furthermore, the Ir–Mo–Rh–Ru–W HEAs with hcp structure11) are also unique in that the alloy compositions are optimized by thermodynamic predictions11) using Thermo-Calc with the TCHEA3 database for HEAs. This implies that one can fabricate Ir–Mo–Rh–Ru–W HEAs with bcc or fcc structure by paying attention to the appropriate VEC values for their structures and composition optimization. In other words, the authors came to believe that the Ir–Mo–Rh–Ru–W system has the ability to provide HEAs with bcc and fcc structures as well as unprecedented bcc+hcp and hcp+fcc structures as stabilized phases when the VEC values and compositions are optimized.
The purpose of this study was to examine the presence of single bcc, fcc, and hcp structures and plural phases in the Ir–Mo–Rh–Ru–W alloy system in accordance of an alloy design based on VEC analysis and thermodynamic calculations and optimizations.
In the present study, the capital and lower-case letters were intentionally distinguished for denoting the structures clearly between the predictions and experiments including conventional descriptions, respectively. For instance, “BCC_A2,” “FCC_A1,” and “HCP_A3” were used for the predictions with Thermo-Calc, whereas lower-case letters, “bcc,” “fcc,” and “hcp” were given for the experiments and conventional descriptions.
2.1 Alloy designAn Ir–Rh–Ru–W–Mo alloy system was investigated experimentally as well as computationally in accordance with an alloy design. The significant point of the present alloy design, from a computational aspect, was to try to identify a compositional line on which all the possible three structures of bcc, hcp, and fcc appear at a given temperature. In determining the composition line, the authors referred to a relationship between the VEC value of the structure12,14) and computational calculations of the cross-sectional phase diagram by the CALPHAD scheme before starting the experiments. An underlying concept of the sandwich strategy11) for the Ir–Mo–Rh–Ru–W system was adopted in selecting the Ir–Rh–Ru–W–Mo system. That is, Ru with a VEC of 8 is put in Mo and W with VEC = 6 and Rh and Ir with VEC = 9 in the periodic table. As a result of trial and error, as described in the Appendix, the alloy design eventually led to selecting Ir0.415254(100−2x)Rh0.415254(100−2x)Ru0.169492(100−2x)WxMox alloys, which involved five representative alloys (Alloys A–E). Specifically, the alloy compositions were determined by trial and error to meet the requirement that the VEC values of the alloys vary approximately in the range of 6.8–8.5 by referring to empirically and statistically obtained data12) relating the VEC and types of structures: VEC < 6.87 (bcc), 6.87 ≤ VEC < 8.0 (bcc+fcc), and VEC ≥ 8 (fcc). Table 1 summarizes the compositions of Alloys A–E, their VEC values, and their configuration entropies normalized by the gas constant (Sconfig/R). The values of Sconfig/R of Alloys A, D, and E are slightly smaller than 1.5, such that these three alloys cannot be HEAs by the strict definition. Furthermore, the contents of Ir and Rh in Alloy E are not in the range of 5 ≤ ci/at% ≤ 35, and thus, this alloy cannot be a HEA according to the strict definition. However, the present study regards Alloys A–E as HEAs based on the definition of HEAs in a wide sense.

The cross-sectional phase diagram calculated with Thermo-Calc 2019a with the TCHEA3 database is shown in Fig. 1, which includes Alloys A–E on a composition line. In the computations, only the following phases from solutions (LIQUID, FCC_A1, BCC_A2, BCC_B2, and HCP_A3) and chemically ordered fcc- and bcc-family solid solutions (FCC_L12 and BCC_B2) were considered in the calculations because of the restriction on the number of phases in the computations. Here, preliminary investigation revealed that both FCC_L12 and BCC_B2 phases were calculated in disordered states, and thus, they exactly corresponded to FCC_A1 and BCC_A2, respectively. However, the absence of the other conventional intermediate or intermetallic compounds in the calculation results was confirmed separately for Alloys A–E over the temperature range shown in Fig. 1. The non-appearance of compounds is a nature of the Ir–Rh–Ru–W–Mo alloy system, as presented in a previous report.11) Figure 1 predicts that Alloys A, C, and E, respectively, will form a single bcc, hcp, and fcc phase at high temperatures, such as T = 2100 K, whereas Alloys B and D will be obtained as dual phases of bcc+hcp and hcp+fcc, respectively. Furthermore, Alloys A–E have the ability to be formed into triple phases of bcc+hcp+fcc and bcc+fcc phases with decreasing annealing at low temperatures. This variety of phases that may appear on a cross-sectional phase diagram is a significant feature of the Ir–Rh–Ru–W–Mo System. These computationally predicted phases were examined experimentally.
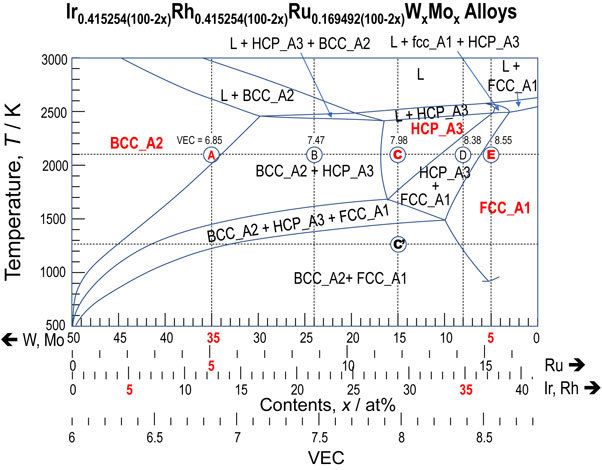
Cross-sectional phase diagram calculated with Thermo-Calc 2019a and the TCHEA3 database along a composition line denoting the Ir0.415254(100−2x)Rh0.415254(100−2x)Ru0.169492(100−2x)WxMox alloys. The symbols A–E and C′ indicated by circles denote the annealing temperature of Alloys A–E. The horizontal axes represent the contents of W and Mo as bcc former, those of Ru as hcp former, and those of Ir and Rh as fcc former, together with the VEC of the alloys.
Additionally, a property diagram that displays the amounts of all phases as a function of temperature was computed for Alloys A–E to compensate for the sparsity of Fig. 1 in terms of the phases considered under the restriction. In calculating the property diagrams, all the possible phases, including intermetallic/intermediate compounds, were considered where these phases were derived from a default condition after selecting the constituent elements of Ir, Rh, Ru, Mo and W. The property diagrams shown in Fig. 2 indicate the non-appearance of other phases, except for bcc, fcc, hcp, and liquid over a wide temperature range from 500 to 2500 K in Alloys A–E, supporting results in Fig. 1 calculated under limited conditions by considering LIQUID FCC_A1, FCC_L12, BCC_A2, BCC_B2, and HCP_A3 only.

Property diagrams calculated with Thermo-Calc 2019a and the TCHEA3 database for Alloys A–E by considering all the possible phases, including the intermetallic/intermediate compounds from a default condition, which were determined by selecting the constituent elements of Ir, Rh, Ru, Mo, and W. The vertical broken lines correspond to the annealing temperatures for comparison.
Alloy ingots of ∼5 g with nominal compositions of the Ir0.415254(100−2x)Rh0.415254(100−2x)Ru0.169492(100−2x)WxMox alloys (Alloys A–E: x = 35, 24, 15, 8, 5) were prepared by arc melting from raw metals with industrial purity. The alloy compositions are summarized in Table 1. The raw metals were commercially obtained and had a purity of 99.9 mass%. The Ir, Rh, and Ru elements, which had an initial form of powders, were separately consolidated in a bulk form prior to alloying. The 5-g samples were formed into button-shaped ingots of ∼10 mm diameter and ∼5 mm height. The samples were annealed at a high temperature to confirm the equilibrium phases. The as-prepared ingots were annealed with a magnesium oxide crucible as a contacting material in a high-temperature furnace with a graphite heater. The chamber of the furnace was vacuumed (∼10−2 Pa) in advance and then filled with high-purity Ar gas of ambient pressure. The samples were homogenized by annealing, followed by cooling in the furnace. The cross-sections of these alloys, which were cut into two pieces perpendicular to the base, were examined for their structure by X-ray diffraction (XRD). In addition, the samples were observed for their morphology by scanning electron microscopy (SEM), and the chemical composition was analyzed by energy-dispersive X-ray spectroscopy (EDX) equipped on the SEM.
Alloys A–E annealed at 2100 K for 2 h and Alloy C annealed at 1273 K for 200 h were analyzed with XRD for their crystallographic structures. The XRD profiles shown in Figs. 3(c), (e) indicate that Alloys C and E annealed at 2100 K exhibit reflections consistent with a single hcp phase and a single fcc phase, respectively. These identified phases are denoted with Miller indices. Figures 3(c), (e) for Alloys C and E indicate the reproducibility of the predictions shown in Fig. 1. However, Alloy D does not provide the mixture of hcp+fcc structures, but forms into a single hcp phase as shown in Fig. 3(d). This disagreement between the prediction and experiment for Alloy D was presumably due to a narrower composition region of hcp+fcc predicted in Fig. 1. The authors did not investigate further to identify the intermediate alloy composition between Alloys D and E that exhibits hcp+fcc at 2100 K. This is because the accurate assessment of the cross-sectional phase diagram is not the purpose of the present study. On the contrary, the authors strongly believe that Alloy D with the hcp structure and Alloy E with the fcc structure indirectly prove the presence of a composition region of hcp+fcc between the compositions of Alloys D and E. Thus, it appears that the experiments tended to reproduce the predictions shown in Fig. 1 for Alloys C–E.

XRD patterns measured with Co-Kα radiation for (a)–(e) Alloys A–E annealed at 2100 K for 2 h and (c′) Alloy C annealed 1273 K for 200 h.
In strong contrast, experiments for Alloys A and B exhibited considerably different results compared with the predictions. Specifically, the XRD profiles of Alloys A and B annealed at 2100 K indicated, as shown in Figs. 3(a), (b), a mixture of bcc+hcp phase and a single hcp phase, respectively. These results suggest that the composition region of bcc+hcp and bcc predicted in Fig. 1 should considerably shift to the left, corresponding to the high-fraction direction of the bcc-forming elements of W and Mo. Again, the authors did not perform further experiments to determine the exact alloy compositions that exhibit a single-bcc structure. This is because the present study was performed in a framework of HEAs with the approximate composition of 5 ≤ ci/at% ≤ 35. The above experimental results shown in Fig. 3(a)–(e) reveal that the CALPHAD predictions at 2100 K gave better agreement with experiments for Alloys C–E with greater VEC than ∼8 than for Alloys A and B. Moreover, Alloy C annealed at T = 1273 K for 200 h, as shown in Fig. 3(c′), was not formed into a mixture of structure bcc+fcc as predicted computationally in Fig. 1, but into a single hcp structure. The reason for the non-appearance of the thermodynamically stable bcc+fcc structure in Alloy C annealed at 1273 K will be discussed in the next section. Additionally, Alloys B–D in the as-prepared states were identified as having an hcp structure; their results are not shown in Fig. 3.
An analysis to determine the lattice constants of the bcc and fcc phase (a) and the hcp phase (a, c), as well as their ratio (c/a) was performed for Alloys A–E annealed at 2100 K for 2 h and Alloy C annealed at 1273 K for 200 h (Alloy C′). The results summarized in Table 2 indicate that Alloy A with a partial hcp structure and Alloys B–D with an hcp structure exhibit c/a ranging 1.611–1.593. The values of c, a, and c/a in Alloy C with VEC = 7.981 are almost the same as those of the previous data:11) Ir26Mo20Rh22.5Ru20W11.5 and Ir25.5Mo20Rh20Ru25W9.5 HEAs with hcp structure with VEC ∼ 7.86, and those of pure Ru.15) Moreover, no apparent differences in a, c, and c/a are seen between Alloys C and C′.

The results of further examinations of Alloys C and E annealed at 2100 K, performed by observing their morphology with SEM and by analyzing chemical compositions with EDX, are shown below. Figure 4 presents SEM and EDX images of Alloy C annealed at 2100 K for 2 h, whereas Fig. 5 depicts those of Alloy E. The SEM image in Fig. 4(a) demonstrates that Alloy C appears to be almost homogeneous at a submillimeter scale, except for the presence of grain boundaries indicated by areas with slightly dark contrast. The EDX analysis revealed that these grain boundaries were slightly poor in Ir in Fig. 4(b) and rich in Rh in Fig. 4(c). However, Ru, W, and Mo are homogeneously distributed over the grain boundaries, as shown in Fig. 4(d)–(f). Thus, Figs. 3 and 4 revealed that Alloy C annealed at 2100 K for 2 h were formed into a single hcp structure. Moreover, an analysis of the SEM image and element-mapping images of Alloy E annealed at 2100 K for 2 h, shown in Figs. 5(a) and 5(b)–(f), respectively, demonstrates the formation of a single fcc phase.

(a) SEM image and (b)–(f) element-mapping images of Alloy C annealed at 2100 K for 2 h.

(a) SEM image and (b)–(f) element-mapping images of Alloy E annealed at 2100 K for 2 h.
The possible reasons for the formation of single solid solutions in Alloys B–E, as experimentally observed in the Ir–Rh–Ru–W–Mo system, are rationalized in terms of the geometrical features of the liquidus and solidus lines/temperatures in the cross-sectional phase diagram shown in Fig. 1. Furthermore, a feature of Alloy E as a HEA with hcp structure was highlighted by the analysis of the Gibbs free energy (G).
The present results are significant from a viewpoint of the geometrical features of liquidus and solidus lines, in that Alloy E is a new class of refractory HEA with fcc structure. In particular, it is worth emphasizing that the liquidus temperature (Tl) of Alloy E reaches ∼2600 K, which is approximately 1000 K higher than that of the CrMnFeCoNi HEA.16) According to Fig. 1, the composition of Alloy E shows a narrow range of the L+hcp_A3 region, nearly as narrow 100 K, followed by a relatively small temperature range of the hcp_A3+fcc_L12 phase region above 2200 K. These narrow composition regions made it possible to form a single fcc structure during its solidification from a melt. A similar situation could be observed in Alloys C and D when they solidified from their melts. The narrow temperature range between Tl and Ts (ΔTl−s) over the compositions of Alloys C–E is considerably important to obtain a single solid solution. This importance was pointed out in the authors’ previous work.6) This alternatively supports the disagreement of Alloys A and B with relatively wide ΔTl−s of 200 K or more. The large ΔTl−s of Alloys A and B led to them forming other phases in the experiments with respect to the predictions.
The formation of a HEA with fcc structure in Alloy E in the Ir–Rh–Ru–W–Mo system was analyzed in detail with a G-composition diagram, as shown in Fig. 6. Alloy E is unique as a HEA with fcc structure, in that the difference in G between the FCC_A1 and HCP_A3 phase (ΔGHCP_A3–FCC_A1) is smaller than 1 kJ/mol, as depicted in Fig. 6. Further calculations were performed to examine the temperature dependence of G of LIQUID, BCC_A2, FCC_A1, and HCP_A3 structures for Alloy E. Figure 7(a) exhibits the conventional tendency that G increases with decreasing T. Also, Fig. 7(b) indicates that ΔGHCP_A3–FCC_A1 increases with decreasing T. The extrapolated value of ΔGHCP_A3–FCC_A1 was evaluated to be 4.5 kJ/mol, which roughly corresponds to ΔGhcp–fcc values of SGTE of pure elements of fcc formers:17) 3.00 kJ/mol (Rh) and 4.00 kJ/mol (Ir). Alloy E exhibited somewhat high value of ΔGHCP_A3–FCC_A1 at low temperature, such as ΔGHCP_A3–FCC_A1 = 3.9 kJ/mol at T = 300 K. Thus, it was found that Alloy E exhibits small value of ΔGHCP_A3–FCC_A1 < 1 kJ/mol at high temperature range around 2000 K. This small value of ΔGHCP_A3–FCC_A1 at high temperature range suggests that Alloy E would possess an extremely low stacking fault energy and that it would tend to exhibit a mixed structure of hcp+fcc when it was mechanically tested at elevated temperatures. In other words, such a small difference in ΔGHCP_A3–FCC_A1 of Alloy E will lead to transformation-induced plasticity (TRIP),18) which includes a lamellar hcp phase in the fcc matrix containing high-density stacking faults. Similarly, Alloy E will contain twins introduced during deformation and will possess low stacking fault energy (SFE). Thus, the Ir0.415254(100−2x)Rh0.415254(100−2x)Ru0.169492(100−2x)WxMox system has great advantage over the CrMnFeCoNi HEA, in that the hcp phase is in a thermodynamically stable state without compulsory loading of high pressure, and that ΔG between the hcp and fcc structures can simply be analyzed with the CALPHAD scheme.

Gibbs free energy (G) calculated with Thermo-Calc 2019a and the TCHEA3 database at T = 2100 K along the cross-sectional composition line of Ir0.415254(100−2x)Rh0.415254(100−2x)Ru0.169492(100−2x)WxMox, which includes Alloys A–E.

(a) Temperature dependence of Gibbs free energy (G) of LIQUID, BCC_A2, FCC_A1, and HCP_A3 structures calculated for Alloy E with Thermo-Calc 2019a and the TCHEA3 database and (b) difference in G between FCC_A1 and HCP_A3 structures (ΔGHCP-A3-FCC_A1).
First, the agreement and disagreement between the experimental and computational data on the structure of Alloys B–D are discussed by focusing on the formation of HEAs from thermodynamic viewpoints and the applicability of TCHEA3 database. Then, the effect of the VEC on the formation of HEAs without and with hcp structure is discussed with respect to conventional HEAs comprising 3d transition metals and the present alloys in the Ir–Rh–Ru–W–Mo system.
4.1 Thermodynamic viewpoints and applicability of TCHEA3 databaseThe present experiments revealed that HEAs with a single hcp structure were formed in Alloys B–D annealed at 2100 K for 2 h and Alloy C annealed at 1273 K for 200 h. These results indicate that the hcp structure in the Ir–Rh–Ru–W–Mo system might exist more widely than thermodynamically expected. Such disagreements between the experimental and computational results at low temperature range are also reported in the Ir26Mo20Rh22.5Ru20W11.5 and Ir25.5Mo20Rh20Ru25W9.5 alloys11) in the authors’ previous study. Specifically, these two alloys,11) both annealed at 2373 K and as-prepared by arc-melting, formed into a single hcp structure, although the calculations with Thermo-Calc suggested the co-existence of bcc and fcc with hcp structures at temperatures lower than 1300 K. The presence of the stable phase in the high temperature range is rational in HEAs, because of the high-entropy effect. That is, the high entropy (S) term due to high-entropy effect accompanied by a high absolute temperature (T) environment overcomes the enthalpy term (H) in the Gibbs free energy, as expressed by G = H − TS, leading to a reduction in G and stabilized solid solutions.
However, the experimental data of Alloys B–D as well, as the previously reported Ir–Rh–Ru–W–Mo HEAs, with hcp structure11) in the low temperature range, present an ironic problem. That is, the strong tendency to form an hcp structure might not be controlled intentionally to produce bcc and fcc structures, although they were predicted in the thermodynamic calculations. From thermodynamic viewpoints, it appears that the formation of the structure of Alloys B–D may be affected by imperfections of TCHEA3 database19) when applying it to the multicomponent Ir–Rh–Ru–W–Mo system for the following sub-ternary and sub-binary systems. Specifically, only the Mo–Ru–W ternary system is tentatively assessed19) among sub-ternary systems and Mo–Ir, W–Ir, Mo–Rh, and W–Rh binary systems are not19) assessed in the full range of composition and temperature. Thus, these binary systems were computed with a template of Property Diagram by selecting Phase Diagram in Calculation Type as shown in Fig. 8. Features of Fig. 8 showed the absence of the HCP_A3 phase and the overestimation of maximum solid solubility (maximum amount of primary solid solubility). Specifically, Fig. 8 indicates that the calculated binary phase diagrams did not contain HCP_A3, but just contained BCC_A2 and FCC_A1 and their mixture. However, the maximum solid solubilities shown in Fig. 8 were overestimated, particularly in the BCC_A2 sides. In details, the maximum solid solubility of the BCC_A2 structure was calculated to be as large as approximately 30 at% of solute or more. The maximum solid solubility of the FCC_A1 structure was also large, and in the range of approximately 15–30 at% of solute. According to phase diagrams,20) the maximum solid solubility of the Ir–Mo, Ir–W, Mo–Rh, and Rh–W binary systems should be approximately 5–20 at% and 15–22 at% of solute for bcc_A2 and fcc_A1, respectively.

Calculate binary phase diagrams of (a) Ir–Mo, (b) Ir–W, (c) Mo–Rh, and (d) Rh–W systems with TCHEA3 database where Calculation Type was set to be Property Diagram: these binary systems are not19) assessed by TCHEA3 database in the full range of composition and temperature, and thus, they were not computable with Binary Calculator as Graphical Mode Activity.
As described above, absence of the hcp structure and overestimation of solid solubility — in particular, at bcc former side — should affect the assessment of the Ir–Rh–Ru–W–Mo system. The former directly affected the smaller area of HCP_A3 in Fig. 1 than that in the experiments. However, the latter also affected the shift in the area of BCC_A2 in Fig. 1. In reality, the latter showed that the prediction of Alloy A at 2100 K as BCC_A2 instead of bcc_A2+hcp_A3 was experimentally denied and that better reproducibility of experiments at 2100 K was observed for Alloys C–E than for Alloys A and B. Furthermore, the former and latter affected the disagreement between the prediction and experiments for Alloy C′ at low temperature. Specifically, the prediction shown in Figs. 1 and 2 revealed that Alloy C′ (Ir29.0678Rh29.0678Ru11.8644W15Mo15) decomposed into BCC_A2 (Ir3.835Rh15.368Ru0.802W45.119Mo34.876) and FCC_A1 (Ir35.341Rh32.474Ru14.615W7.512Mo10.058) as summarized in Table 3. The tie line at its equilibrium is not present on the composition line of the cross-sectional phase diagram shown in Fig. 1 because of the nature of Alloy C′ from the multicomponent system. The compositions of the equilibrated phases of Alloy C′ at 1273 K shown in Table 3 indicate that the BCC_A2 was poor in Ir and Ru and rich in W and Mo by composition differences of 14 at% or more. However, the FCC_A1 was slightly poor in W and Mo and rich in Ir and Mo but the differences were nearly 5 at% or smaller. Consequently, it was considered that the larger difference in compositions between the BCC_A2 and Alloy C′ made it difficult to equilibrate, because of the large composition modulation. Hence, it is tentatively concluded that the disagreements between the experiments and calculations in Fig. 1 are principally affected by the shortcomings of the TCHEA3 database. A supplementary, slow diffusion effect in the Ir–Rh–Ru–W–Mo system at a lower temperature range would affect the disagreement of Alloy C′, which required large composition modulation to achieve equilibrium (BCC_A2+FCC_A1).

The conventional VEC analysis reported by Guo et al.12) indicates that bcc, bcc+fcc, and fcc are stable in HEAs when VEC < 6.87 (bcc), 6.87 ≤ VEC < 8.0 (bcc+fcc), and VEC ≥ 8 (fcc). This analysis reported in 2011 does not contain the hcp structure, as the first HEAs with the hcp structure6) were presented in 2014. It has recently been reported that the hcp structure is stable at VEC = 36) for lanthanide alloys, VEC = 2.821) for light-weight elements,8) and VEC ∼ 7.86 for Ir26Mo20Rh22.5Ru20W11.5 and Ir25.5Mo20Rh20Ru25W9.5 alloys;11) moreover, VEC = 7.472–8.378 for Alloys B–D in the present study. These VEC values of HEAs with hcp structure of ∼3 and ∼7–8 act as a guiding principle derived by Miedema’s model13,14) for structural stability and the Friedel model for the number of d-electrons (nd)22) in the ranges 2.6 < nd < 3.5 and 6.5 < nd < 7.4. Here, it should be noted that these models are valid for the paramagnetic elements. Accordingly, the VEC analysis based on these models provides rational results for refractory HEAs with bcc structure23,24) with VEC ∼ 5. In general, the VEC analysis given by Guo et al. is correct, as a result of the statistical analysis that combined the structures of HEAs with the VEC values. However, the VEC analysis given by Guo et al. for the Cantor alloy,2,25) as a HEA with fcc structure, should be treated with care because of the inclusion of the ferromagnetic constituents of Fe, Ni, and Co. For instance, if Fe with VEC = 8 were a paramagnetic element such as Ru and Os with VEC = 8, the VEC analysis given by Guo et al. would provide slightly different threshold values of VEC for the boundary between bcc+fcc and fcc structures. Consequently, the VEC analysis given by Guo et al. should be modified by including the experimentally confirmed VEC ranges for HEAs with hcp structure under special supplementary conditions. The present results showed that the supplemental conditions are that HEAs with hcp structure are composed of 4d and 5d transition metals and these alloys are subjected to high-temperature annealing near the solidus temperature or solidified from a melt. Thus, the supplemental VEC analysis should include 7.5 ≤ VEC ≤ 8.4 as well as VEC ∼ 3 for HEAs with hcp structure. In particular, the former supplemental VEC analysis, 7.5 ≤ VEC (hcp) ≤ 8.4, does not contradict the VEC analysis given by Guo et al. as VEC < 6.87 (bcc), 6.87 ≤ VEC < 8.0 (bcc+fcc), and VEC ≥ 8 (fcc). This is because Alloy C annealed at 1273 K for an extremely long time leaves scope for the formation of the bcc+fcc structure, as shown in Fig. 2, when the slow diffusion is overcome to yield a thermodynamic equilibrium state.
Five multicomponent alloys (Alloys A–E) on a composition line, Ir0.415254(100−2x)Rh0.415254(100−2x)Ru0.169492(100−2x)WxMox (x = 35, 24, 15, 8, and 5 at%) were investigated experimentally for their phase stability according to computational predictions with Thermo-Calc and the TCHEA3 database. The experiments revealed that the samples annealed at 2100 K for 2 h had a mixed dual-phase bcc+hcp structure in Alloy A, single hcp structure in Alloys B–D, and single fcc structure in Alloy E. CALPHAD predictions gave better agreement with the experiments for Alloys C–E, with greater VECs of ∼8, than for Alloys A and B. A refractory HEA with fcc structure was newly found in Alloy E. Alloy C annealed at T = 1273 K for 200 h retained its hcp structure instead of the predicted bcc+fcc phases. The formation of an hcp structure in the Ir–Rh–Ru–W–Mo system could be affected thermodynamically by the necessity of large composition modulation to achieve bcc+fcc phases and kinetically by slow diffusion particularly in the relatively low temperature range. The disagreements between the predictions and experiments were principally because the Ir–W, Ir–Mo, Rh–W, and Rh–Mo binary systems were not assessed in the full range of composition and temperature by the TCHEA3 database, leading to smaller HCP_A3 and a shift in BCC_A2 to the HCP_A3 side in the predictions. A VEC analysis has been modified to compensate for the lack of data on HEAs with hcp structure by adding 7.5 ≤ VEC ≤ 8.4, as well as VEC ∼ 3. The former modification of the VEC analysis is valid for HEAs comprising 4d and 5d transition metals and the higher temperature range near the limit of the solid phase.
The composition line has been determined under the following five conditions.
The specific procedure to determine the composition line was as follows.
First, the exact equiatomic composition (Ir20Rh20Ru20W20Mo20, at%) was set up to be the initial composition, which was included in the initial composition line.
Second, Cases 1–3 were tested preliminarily by calculating cross-sectional phase diagrams. As a result, it was revealed that Case 1 met the demand in terms of conditions 1 and 2. The initial composition line for Case 1 included Ir20Rh20Ru20W20Mo20, and W50Mo50, and Ir33.333Rh33.333Ru33.333 at the ends in the pseudoternary system.
Third, the composition line was modified slightly by giving a modified composition that deviated by approximately 2–3 at% from the Ir20Rh20Ru20W20Mo20. Specifically, the modified composition was determined by changing the ratios of the contents of bcc, hcp, and fcc formers from the initial ratio of 2:1:2 for Ir20Rh20Ru20W20Mo20. This process was swiping the compositions (Swipe-1), as shown in Fig. A1(b). Then, the cross-sectional phase diagram was computed along the modified composition line.
Fourth, the calculated phase diagrams containing the initial and modified composition were compared in terms of the following two parameters: (i) the composition gap between the phase boundaries of bcc/bcc+hcp and hcp+fcc/fcc and (ii) the size of the areas of the bcc, hcp, and fcc phases in the cross-sectional phase diagram. When the composition gap (i) becomes smaller by modifying the composition, further changes of the ratios of the contents of bcc, hcp, and fcc formers were carried out to consider the further modified composition line. This is because of the favorite tendency in terms of condition 2. However, opposite ratios of bcc, hcp and fcc formers were tested when the composition gap (i) becomes larger. The authors also paid attention to (ii) to make the experiments easier. The above trial was repeated in sequence by replacing the relationship between the initial and modified compositions with the modified and second-modified compositions and so forth.
Finally, the contents between the bcc formers (W and Mo) and those between the fcc formers (Ir and Rh) were differentiated to find out the best composition line by referring to condition 2. This process was termed “Swipe-2” in Fig. A1(b).

(a) Procedure to determine the preferability among Cases 1–3 to achieve bcc, bcc+hcp, hcp, hcp+fcc, and fcc structures in a composition line by regarding the Ir–Rh–Ru–W–Mo quinary system as a pseudoternary system consisting of bcc–hcp–fcc formers by degenerating W and Mo into bcc-former and Ir and Rh into fcc-former and (b) after determining Case 1 as the most favorable by process of Swipe-1, the appearance of bcc, bcc+hcp, hcp, hcp+fcc, and fcc structures in a composition line was optimized by changing the ratio between the contents of W and Mo and those of Ir and Rh (Swipe-2).
This work was supported by JSPS KAKENHI Grant Number JP17H03375.