2019 Volume 60 Issue 2 Pages 338-345
2019 Volume 60 Issue 2 Pages 338-345
We have examined solid solubility extension for melt-spun Cu–Ti alloys (4, 6, 7, and 8 mass% Ti) in comparison with that for conventional quenched alloys. X-ray diffraction (XRD) measurements revealed that the solid solubility extension for the melt-spun alloys was in the range of 7–8 mass% Ti, whereas that for quenched alloys was less than 6 mass% Ti. After annealing the melt-spun alloys at 673 K, the XRD measurements revealed sidebands with no intermetallic compound peaks, bright-field transmission electron microscopy images showed modulated structures, and selected-area diffraction patterns exhibited satellite structures; taken together, these experimental results confirm the occurrence of spinodal decomposition. In this study, however, distinct superlattice reflections were not observed.
Dilute Cu–Ti alloys are mainly used for high-strength conductive springs, diaphragms, and corrosion- and wear-resistant materials owing to their high tensile strength of over 700 MPa, good workability, and stress-relaxation properties.1–6) However, the electrical conductivity of these alloys is less than 15% IACS, where % IACS is the electrical conductivity relative to that of the International Annealed Copper Standard, which is inferior to that of commercial Cu–Be alloys or Cu–Ni–Si alloys.7–13)
The main reason for the relatively low electrical conductivity of Cu–Ti alloys compared with Cu–Be alloys and Cu–Ni–Si alloys is the presence of solute Ti in the Cu matrix. This means that the scattering of the conduction electrons by the impurity potential due to the solute Ti atoms is relatively high. According to Nordheim’s rule combined with Matthiessen’s rule,14) the residual resistivity ρ can be expressed as
| \begin{equation} \rho = Ax(1-x) + \rho_{0} \end{equation} | (1) |
| \begin{equation} \rho \approx Ax + \rho_{0} \end{equation} | (1′) |
Furthermore, Cu–Ti alloys exhibit spinodal decomposition during aging,1,3,4,6,12,18–27) which is an interesting and attractive property from both academic and industrial perspectives. According to Cornie et al.,1) the spinodal decomposition process involves the development of ⟨100⟩ compositional waves and formation of a periodic array of interpenetrating block-like rods along the ⟨100⟩ directions of the matrix, which contributes to the strengthening of age-hardened Cu–Ti alloys; thus, spinodal decomposition is an industrially important phenomenon. Furthermore, measurements of the electrical resistivity of a Cu–4 mass% Ti alloy at 77 K revealed that the electrical conductivity increased from 5.2% IACS to 14% IACS after annealing at 723 K for 1.08 ks,18) which indicates that the electrical conductivity increases with increasing spinodal decomposition. However, more dilute alloys such as Cu–1 mass% Ti have been shown to decompose predominantly by the mechanism of nucleation and growth.28) Additionally, an investigation into the decomposition process of Cu–0.9 at% Ti solid solutions after aging at 623 K by Borchers,29) which was based on the analysis of transmission electron microscopy (TEM) images and the energetics of nucleation aided by CALPHAD calculations, revealed the copious formation of nano-sized coherent ellipsoids or oblong particles from the onset of decomposition. However, Borchers suggested that the occurrence of classical nucleation as the initial step in the decomposition process is essentially precluded if the alloy is supersaturated. Concerning more concentrated Cu–Ti alloys, Cu–5.17 mass% Ti alloy was indeed reported to be spinodal in nature by Laughlin and Cahn.3) Therefore, the Ti content seems to exert a considerable influence on the decomposition process.
Supersaturated states of solute atoms can be achieved by various methods, such as rapid solidification processing (RSP) from the liquid state, mechanical alloying (MA) of solid elements, vapor deposition, laser processing, sputtering, and ion beam mixing.30) In particular, RSP and MA are frequently used to form non-equilibrium phases such as amorphous alloys,31–34) quasicrystals,35–38) and supersaturated solid solutions.
Regarding the application of MA to the formation of supersaturated solid solutions, Xi et al. investigated the extension of the solid solubility of Mo in Cu.39) The solid solubility of Mo in the Cu matrix was found to be less than 4.3 at% upon MA, whereas the equilibrium solid solubility of Mo is less than 0.06 at% even at the higher temperature of 1356 K. Lei et al. investigated the solid solubility of Nb in Cu and found that it could be extended to more than 7 at%,40) whereas Mula et al. demonstrated that 7.5 at% Nb could be dissolved in the Cu matrix on the basis of X-ray diffraction (XRD) analysis and the Gibbs free energy change.41) These two reports indicate that the solid solubility extension of Nb is in the range of 7–7.5 at%,40,41) which is considerably higher than the equilibrium solid solubility of Nb of approximately 0.1 at%42) and indicates that MA greatly increases the solid solubility of Nb in the Cu matrix.
With respect to the use of RSP for the formation of supersaturated solid solutions, Bell and Davies reported the solid solubility extension in Cu–V alloys.43) The solid solubility of V in Cu was found to increase from the equilibrium value of 0.17 at% to 0.5 at%, as determined by electrical resistivity measurements. Consequently, both the MA and RSP approaches seem to possess great potential for extending the solid solubility limit. Therefore, in this study, we investigated the solid solubility extension of Cu–Ti alloys produced by the RSP technique of melt-spinning using XRD and TEM analysis, with a focus on solubility extension and spinodal decomposition.
In addition, as stated above, the Ti content exerts a considerable influence on the alloy decomposition process. However, the maximum dissolved Ti content appears to be limited to approximately 5 mass% for samples fabricated by conventional methods. Therefore, it remains unknown whether more concentrated Cu–Ti alloys can also undergo spinodal decomposition; thus, it seems very intriguing and important to study the decomposition process for more concentrated and more supersaturated Cu–Ti alloys fabricated by the RSP technique of melt-spinning.
Small pieces of oxygen-free copper (99.99%) and titanium (99.99%) were combined in various weight ratios (4, 6, 7, or 8 mass% Ti) and melted in a high-frequency furnace under argon gas shielding. The alloy ingots were fabricated in an alumina crucible, however the reaction between the Ti and the crucible was not significant. Samples (9 × 9 × 0.6 mm, 0.4 g) were cut from the alloy specimens and annealed at 1163 K for 10.8 ks followed by quenching in iced water (a solution treatment with no further annealing processes).
Melt-spun specimens were fabricated under an argon gas atmosphere using a single-roller melt-spinning apparatus with a quartz nozzle. The melt-spun samples (3 g) were quenched from the liquid state at approximately 1523 K with a surface velocity of 42 m/s and a blow-off pressure of 0.6 atm (As-Q samples). The melt-spun samples had a thickness of 50–60 µm and a width of 1–1.5 mm and were aged at 673 K for 0 (As-Q), 1.8, 3.6, or 7.2 ks. After liquid quenching, small amounts of residue were observed in the quartz nozzle, which may indicate reaction between the nozzle and Ti.
Additionally, the melt-spun samples with 7 and 8 mass% Ti were aged at 773 K for 1.8, 3.6, or 7.2 ks to examine the precipitation at a higher aging temperature.
XRD analysis was performed using a Cu-Kα radiation source to assess whether sidebands and new ordered phases had formed during aging. In particular, to detect the presence of sidebands corresponding to the 200 planes, step scanning was performed from 47° to 53° at a scanning speed of 0.3°/min, based on the scanning speed of 0.25°/min reported by Findik.44)
TEM analysis was performed for the melt-spun samples of the Cu–6 mass% Ti alloy aged at 673 K for 3.6 ks and the Cu–7 mass% Ti alloy aged at 673 K for 3.6 ks and 7.2 ks to examine the spinodal decomposition process. Discs with a diameter of approximately 3 mm were punched from the samples and polished by ion milling. High-resolution TEM (JEM-2010, JEOL) operating at 200 kV was then used to observe the microstructures and conduct selected-area diffraction pattern (SADP) analysis. In the SADP measurements using a CCD camera, two exposure times were used: one for the satellite structures (0.1 s) and another for the superlattice reflections (1.0 s). As noted by Zhao and Notis,45) SADP results are dependent on the exposure time. A short exposure time is preferred for measuring spinodal satellites because the bright fundamental reflections obtained after long exposure times tend to wash out the satellites. In contrast, a long exposure time is required to observe superlattice reflections.
Figure 1 shows a partial phase diagram for the Cu–Ti binary system,46) which implies the possibility of Cu4Ti precipitations with increasing Ti content. Concerning the Cu4Ti precipitations, two ordered phases have been reported. One is a metastable coherent precipitate known as the metastable α-Cu4Ti phase with a tetragonal D1a structure (a = 0.587 nm and c = 0.365 nm, prototype, Ni4Mo; space group, I 4/m),3,4) and the other is a stable β-Cu4Ti phase with an ordered orthorhombic structure (a = 0.4528 nm, b = 0.4345 nm and c = 1.2932 nm, prototype, Au4Zr; space group, P nma), which is formed in the grain boundaries during prolonged aging.3,4)
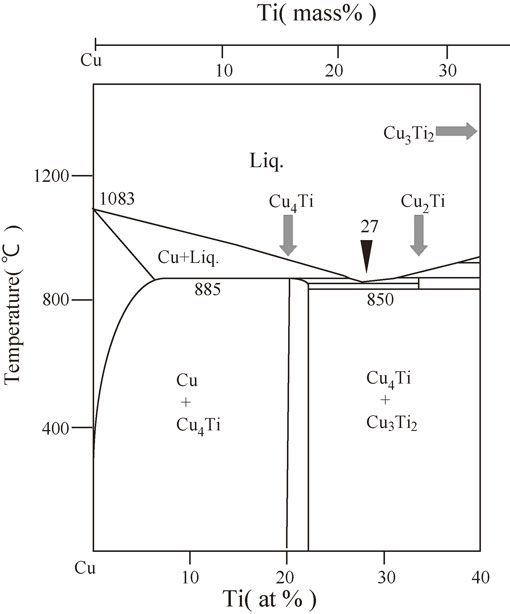
Partial phase diagram for the Cu–Ti binary system.
Figure 2 shows the XRD spectra of the quenched samples of As-Q states from 30° to 80°. For the quenched Cu–6 mass% Ti sample, a peak corresponding to the 115 reflection of the stable β-Cu4Ti phase was observed at a 2θ value of approximately 45°, whereas no peaks except Cu phases were observed in the Cu–4 mass% Ti sample. As predicted from Fig. 1, a Cu4Ti phase—specifically, the stable β-Cu4Ti phase—appeared at a Ti content of 6 mass%.
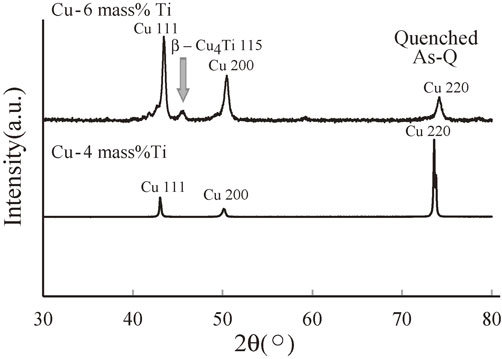
XRD spectra of the quenched samples of the Cu–4 mass% Ti and Cu–6 mass% Ti alloys.
In the early stage of the spinodal decomposition of a Cu–Ti alloy, the supersaturated solid solution of Cu decomposes into two phases, namely, Ti-rich and Ti-lean regions. Subsequently, clusters form in the Ti-rich region and become ordered to generate a metastable coherent α-Cu4Ti precipitate. Upon quenching the Cu–4 mass% Ti alloy, there seemed to be insufficient time to precipitate the stable β-Cu4Ti phase from the Ti-rich region owing to the high cooling rate of about 103 K/s. However, for the Cu–6 mass% Ti alloy, the high cooling rate was not sufficient to prevent the formation of the stable β-Cu4Ti phase in the Ti-rich region. Furthermore, as illustrated in Fig. 1, two phases (Cu and β-Cu4Ti) appear to coexist even up to 1158 K for a Ti content of 6 mass%. Therefore, in the homogenization treatment applied here (annealing at 1163 K for 10.8 ks prior to quenching in iced water), two phases presumably coexist; consequently, we speculate that the precipitation of the stable β-Cu4Ti phase is due to the equilibrium phase diagram and/or insufficient cooling rate. We conclude that the solid solubility extension of Ti is less than 6 mass% for the quenching process.
According to the XRD data, the stable β-Cu4Ti phase was formed rather than the metastable α-Cu4Ti phase, which was ascribed to the rapid cooling rate. As the metastable α phase is formed at a lower temperature than the β phase, the cooling rate of 103 K/s appears to be too high to allow formation of the α phase.
Figure 3 shows the XRD spectra of the melt-spun As-Q alloys with Ti contents of 4, 6, 7, and 8 mass%. No peaks except those corresponding to the Cu phase were observed when the Ti content was less than 7 mass%; however, a very minor β-Cu4Ti 020 peak was detected in the vicinity of the Cu 111 peak in the spectrum of the Cu–8 mass% Ti alloy. Therefore, we conclude that the solid solubility extension of Ti is less than 8 mass% for samples fabricated using RSP (melt-spinning).
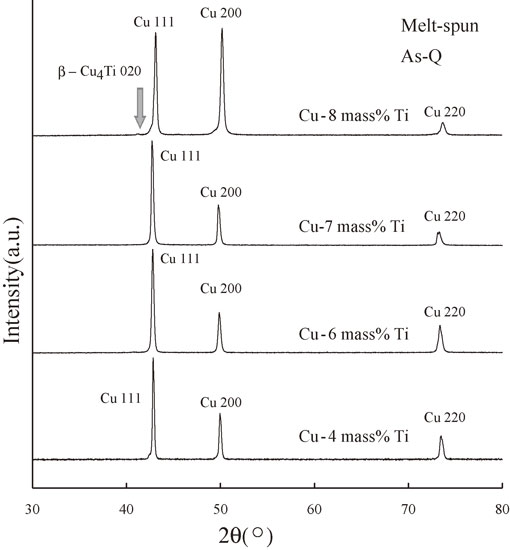
XRD spectra of the melt-spun As-Q samples of the Cu–4 mass% Ti, Cu–6 mass% Ti, Cu–7 mass% Ti, and Cu–8 mass% Ti alloys.
In contrast to conventional quenching, the melt-spinning procedure leads to very rapid quenching of the molten metal at a cooling rate of approximately 8 × 105 K/s,47) which does not permit sufficient time for the single liquid phase to decompose into two phases (Ti-rich and Ti-lean regions). The experimental observation of a slight peak corresponding to the stable β-Cu4Ti phase in the Cu–8 mass% Ti alloy, however, suggests that the rapid cooling rate of about 8 × 105 K/s does not prevent nucleation during the solidification process.
The lattice parameters of the melt-spun As-Q alloys with Ti contents of 4, 6, 7, and 8 mass% are illustrated in Fig. 4 together with those obtained in the work by Saji et al.48) To determine the lattice parameters, we used the program RIETAN-FP.49) From this figure, the lattice parameter was found to be proportional to the Ti content except for the Cu–8 mass% Ti alloy, which means that Vegard’s law holds. By combining Vegard’s law with the XRD results shown in Fig. 3, we can conclude that 7 mass% Ti dissolves in the Cu matrix. In contrast, the lattice parameter of the melt-spun Cu–8 mass% Ti alloy deviates from Vegard’s law. This deviation is presumably attributable to the precipitation of the stable β-Cu4Ti phase as shown in Fig. 3.
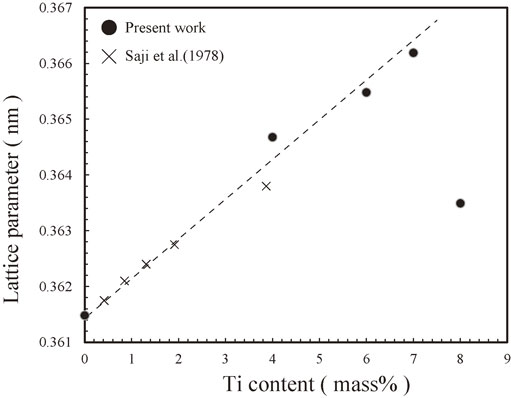
Lattice parameters of the melt-spun As-Q samples of the Cu–4 mass% Ti, Cu–6 mass% Ti, Cu–7 mass% Ti, and Cu–8 mass% Ti alloys together with those obtained in the work by Saji et al.48)
Figure 5 shows the XRD spectra of the melt-spun Cu–6 mass% Ti alloys aged at 673 K for 0 (As-Q), 1.8, and 3.6 ks. Only the Cu phase was observed in all cases, and no peaks corresponding to the metastable or stable Cu4Ti phases (α-Cu4Ti or β-Cu4Ti, respectively) were detected. Therefore, it appears that the annealing temperature of 673 K and annealing time of 3.6 ks are not sufficient for forming these Cu4Ti phases and/or other intermetallic compounds.
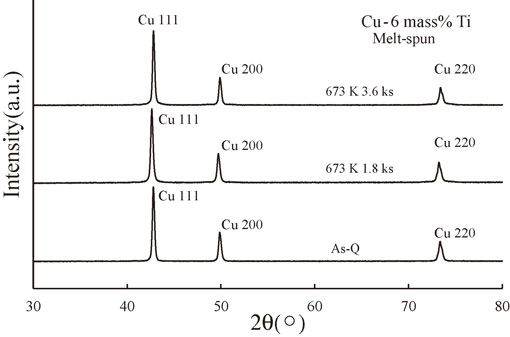
XRD spectra of the melt-spun Cu–6 mass% Ti alloy samples aged at 673 K for 0 (As-Q), 1.8, and 3.6 ks.
Figure 6 shows the step scanning results from 47° to 53° for the melt-spun Cu–6 mass% Ti samples. As indicated by the arrows, sidebands were clearly visible for the sample annealed for 3.6 ks, and slight sidebands were also detected for the sample annealed for 1.8 ks. Therefore, the spinodal decomposition process appears to be favored by longer annealing times.
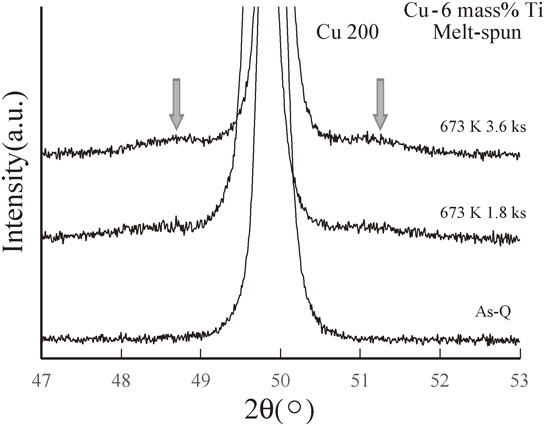
Step scanning results around the Cu(200) diffraction peak for the melt-spun Cu–6 mass% Ti alloy samples aged at 673 K for 0 (As-Q), 1.8, and 3.6 ks.
The wavelength, λ, of the spinodal decomposition was evaluated using the Daniel-Lipson formula:50–52)
| \begin{equation} \lambda = a_{0}\frac{h\tan \theta}{(h^{2} + k^{2} + l^{2})\delta \theta} \end{equation} | (2) |
In an effort to confirm the occurrence of spinodal decomposition, we examined the alloy microstructure by TEM and electron diffraction. Figure 7(a) shows bright-field (BF) TEM images of the melt-spun Cu–6 mass% Ti alloy sample after annealing for 3.6 ks, revealing modulated structures characteristic of spinodal decomposition. Although it is difficult to precisely determine the value of λ, it was roughly estimated to be approximately 5–10 nm, which is in good agreement with the calculated value of 7.8 nm obtained using eq. (2).
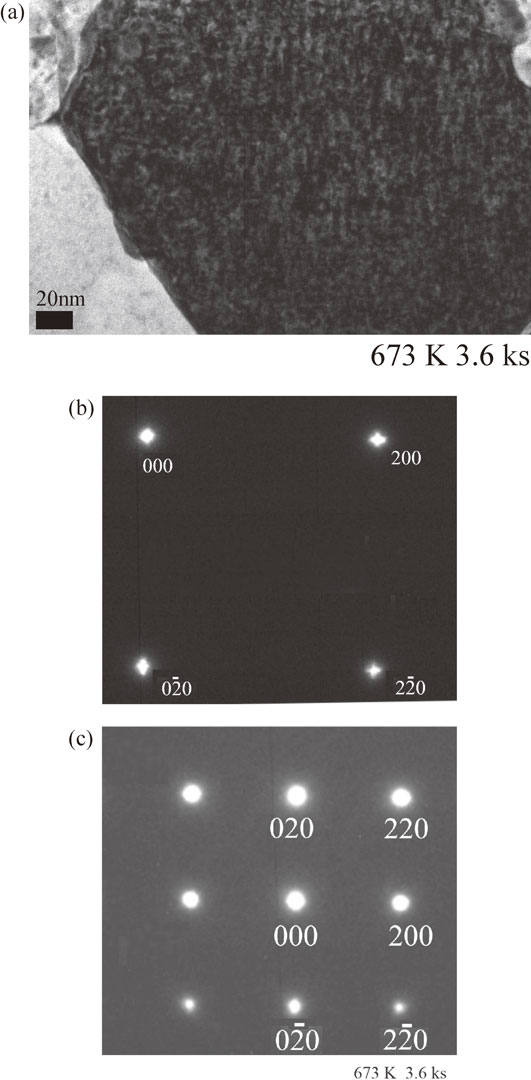
TEM results for the melt-spun Cu–6 mass% Ti alloy sample aged at 673 K for 3.6 ks: (a) BF TEM image, (b) SADP (exposure time 0.1 s), (c) SADP (exposure time 1.0 s).
Figure 7(b) shows an SADP of the same sample with an exposure time of 0.1 s. As expected from Fig. 7(a), distinct satellites caused by structural modulation were observed. Furthermore, it should be noted that the 000 reflection also showed satellites, which is presumably attributable to the short exposure time of 0.1 s and the enhancement of concentration fluctuations. Figure 7(c) shows an SADP of the same sample with an exposure time of 1.0 s, where no long-range ordering (LRO) was observed.
Figure 8 shows the XRD spectra of the melt-spun Cu–7 mass% Ti alloys aged at 673 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks. Similar to the results for the melt-spun Cu–6 mass% Ti alloys, the XRD results for the Cu–7 mass% Ti alloys contained no peaks corresponding to metastable or stable Cu4Ti phases (α-Cu4Ti or β-Cu4Ti, respectively) and only revealed reflections from a single Cu phase, which implies that no precipitates and/or metastable intermetallic compounds were formed even after annealing for 7.2 ks. However, the XRD intensity around the Cu 200 peak appeared to increase slightly for the sample annealed for 7.2 ks, indicating the presence of sidebands.
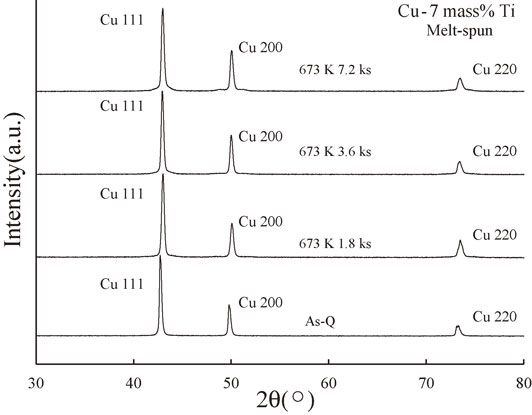
XRD spectra of the melt-spun Cu–7 mass% Ti alloy samples aged at 673 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks.
Figure 9 shows the step scanning results for the melt-spun 7 mass% Ti samples. As indicated by the arrows, sidebands were clearly visible for the samples annealed for 3.6 and 7.2 ks, which suggests the occurrence of spinodal decomposition. Using eq. (2), we obtained λ values of approximately 6.8 and 8.2 nm for the samples annealed for 3.6 and 7.2 ks, respectively; thus, we conclude that spinodal decomposition increased with increasing annealing time.
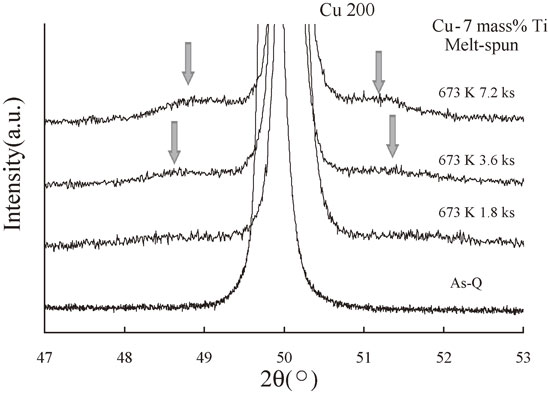
Step scanning results around the Cu(200) diffraction peak for the melt-spun Cu–7 mass% Ti alloy samples aged at 673 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks.
Figure 10(a) shows a BF TEM image of the melt-spun sample of Cu–7 mass% Ti after annealing for 3.6 ks, revealing a modulated structure. Although it is difficult to precisely evaluate the value of λ from this image, we estimate that it is less than 10 nm.
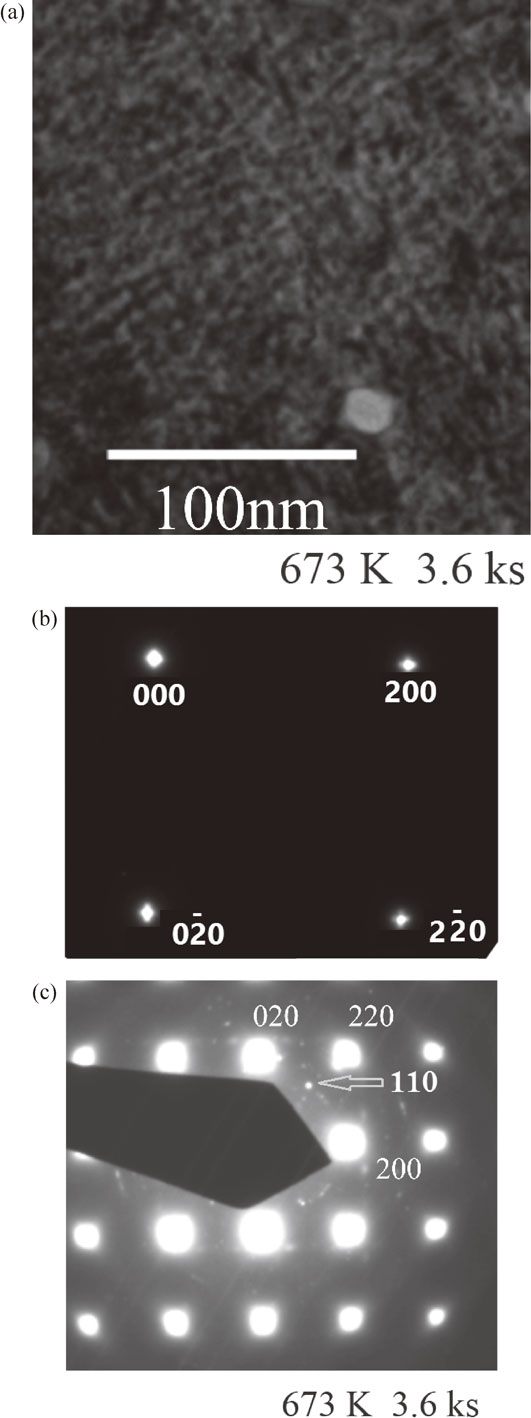
TEM results for the melt-spun Cu–7 mass% Ti alloy sample aged at 673 K for 3.6 ks: (a) BF TEM image, (b) SADP (exposure time 0.1 s), (c) SADP (exposure time 1.0 s).
Figure 10(b) shows an SADP of the same sample with an exposure time of 0.1 s. As in the case of Fig. 7(c), distinct satellites due to structural modulation were observed. Figure 10(c) shows an SADP of the same sample with an exposure time of 1.0 s. Weak spots were detected around the (200), (020), and (220) reflections. Concerning the SADPs of melt-spun Cu–Ti alloys, Batra et al. reported the initial appearance of spots corresponding to the N3M phase, the structure of which can be visualized as a space-filling arrangement of unimolecular clusters of Ni4Mo (D1a) and Ni2Mo (Pt2Mo-type).28) According to this report,28) these spots disappeared upon further aging at 673 K, whereupon D1a superlattice reflections started to increase in intensity; thus, the N3M phase is considered a precursor to the formation of the metastable D1a phase. Our experimental result shown in Fig. 10(c) is entirely different, with no N3M or D1a reflections observed: 1/5{420} and equivalent spots belonging to the two variants of the D1a phase (metastable α-Cu4Ti phase), $\{ 1\ \frac{1}{2}\ 0\} $ and equivalent spots, and $\{ \frac{1}{2}\ \frac{1}{2}\ 0\} $ and equivalent spots of the N3M phase were not present. Instead, 110 spots as indicated by arrow, which do not belong to either the D1a phase or the N3M phase, were clearly visible around the 200, 020, and 220 fundamental reflections. These differences are considered to originate from the differences in Ti concentration or observed area. Consequently, the spots observed in the vicinity of the 200, 020, and 220 reflections are considered to correspond to short-range ordering (SRO) and/or certain oxide reflections, although further analysis and experiments are necessary for definitive identification.
Figure 11(a) shows a BF TEM image of the melt-spun sample of Cu–7 mass% Ti after annealing for 7.2 ks, revealing a modulated structure. From this image, we estimate that the value of λ is approximately 10 nm.
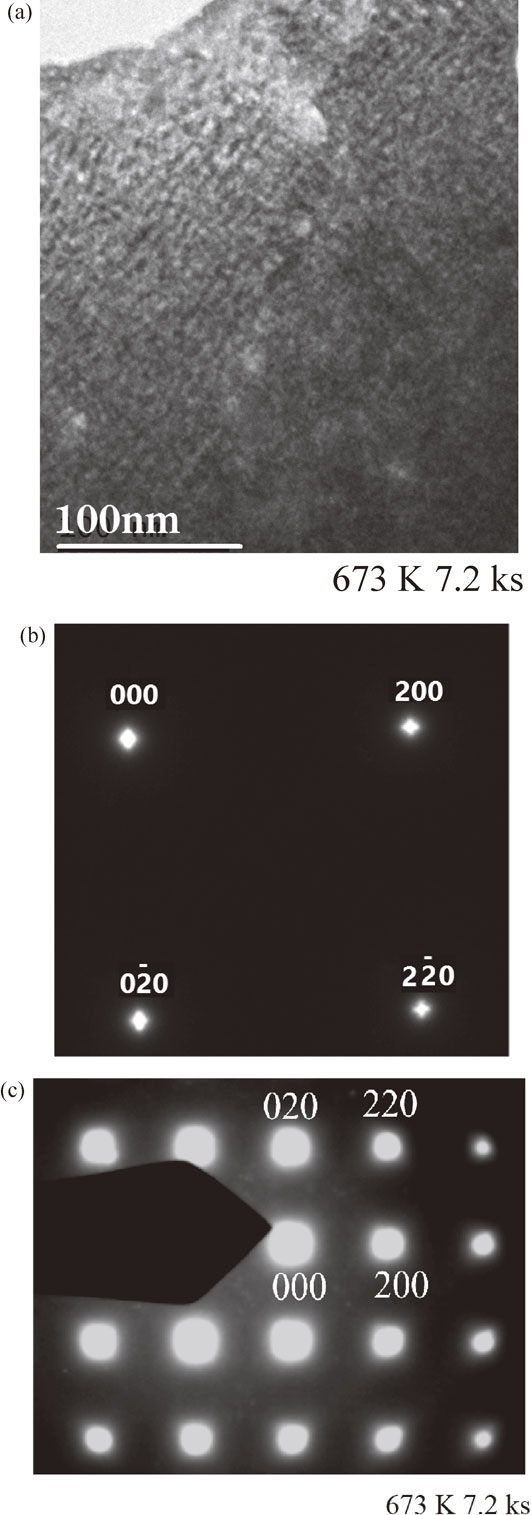
TEM results for the melt-spun Cu–7 mass% Ti alloy sample aged at 673 K for 7.2 ks: (a) BF TEM image, (b) SADP (exposure time 0.1 s), (c) SADP (exposure time 1.0 s).
Figure 11(b) shows an SADP of the same sample with an exposure time of 0.1 s. As in the cases of Figs. 7(b) and 10(b), distinct satellites due to structural modulation were observed. Figure 11(c) shows an SADP of the same sample with an exposure time of 1.0 s, revealing no LRO reflections. Although we were unable to identify the small mist-like spots around 000, these may be due to SRO and/or oxide reflections.
Figure 12 shows the XRD spectra of the melt-spun Cu–8 mass% Ti alloys aged at 673 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks. As discussed previously for Fig. 3, a weak peak corresponding to the stable β-Cu4Ti 020 phase was observed in the vicinity of the Cu111 peak. Despite the detection of the stable intermetallic β-Cu4Ti phase, the intensity of this peak did not appear to increase upon prolonged aging. In contrast, a reflection ascribed to metastable α-Cu4Ti 310 was observed in the vicinity of the Cu 200 peak after annealing for 7.2 ks. Since this phase was observed after an annealing time of 7.2 ks, the metastable α-Cu4Ti phase is considered to grow during the annealing process.
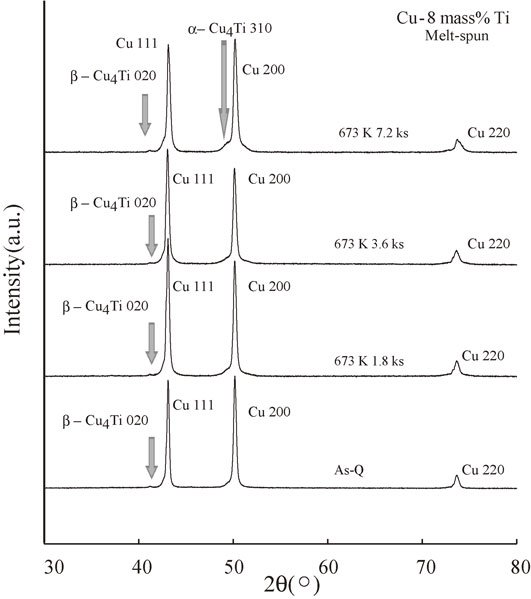
XRD spectra of the melt-spun Cu–8 mass% Ti alloy samples aged at 673 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks.
Figure 13 shows the relative intensities I/I(200), where I(200) denotes the intensity of the Cu 200 reflection, to investigate the growth of the stable β-Cu4Ti phase. Although this comparison is not sufficiently precise to definitively measure the growth of the stable β-Cu4Ti phase with annealing time, the relative intensity of the stable β-Cu4Ti phase appears to remain constant regardless of aging time (the maximum value of I/I(200) is approximately 2% throughout Figs. 13(a)–(d)). Therefore, it appears that the β-Cu4Ti phase does not grow at this annealing time and annealing temperature. One reason for this could be that the stable β-Cu4Ti phase introduced during the cooling process is a high-temperature phase stable above approximately 773 K;53) consequently, the annealing temperature of 673 K is not sufficiently high for the growth of this phase.
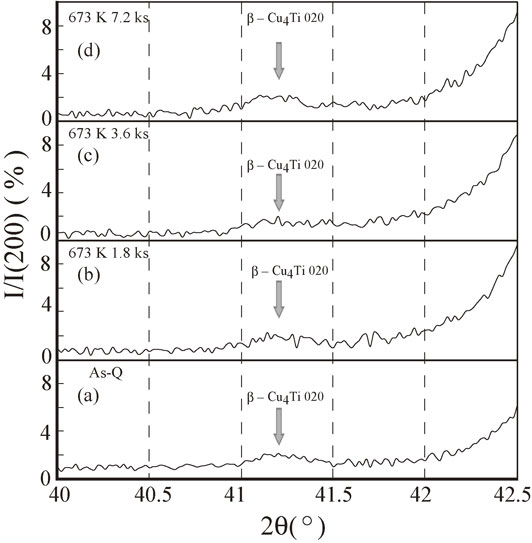
Relative intensities I/I(200) for the melt-spun Cu–8 mass% Ti alloy samples annealed at 673 K for (a) 0 (As-Q), (b) 1.8, (c) 3.6, and (d) 7.2 ks, where I(200) denotes the intensity of the Cu(200) reflection.
Figure 14 shows the XRD spectra of the melt-spun Cu–7 mass% Ti alloys aged at 773 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks. As illustrated in this figure, the metastable α-Cu4Ti phase first appears in the vicinity of the Cu 111 and 200 fundamental peaks after annealing for 1.8 ks. However, the stable β-Cu4Ti phase does not appear before an annealing time of 3.6 ks; thus, the metastable α-Cu4Ti phase would be expected to appear prior to the precipitation of the stable β-Cu4Ti phase. The appearance of such a new phase was never observed, even for the sample aged at 673 K for 7.2 ks (see Fig. 8). Consequently, a higher aging temperature easily promotes the precipitation of the metastable α-Cu4Ti phase and the stable β-Cu4Ti phase.
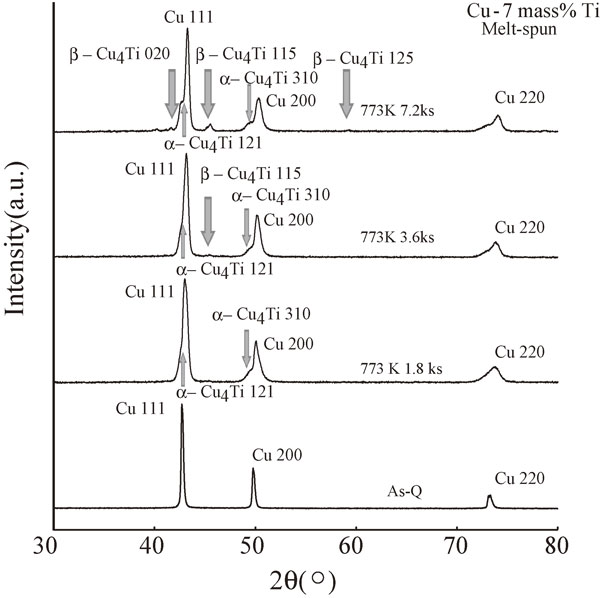
XRD spectra of the melt-spun Cu–7 mass% Ti alloy samples aged at 773 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks.
Figure 15 shows the XRD spectra of the melt-spun Cu–8 mass% Ti alloys aged at 773 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks. In contrast to Fig. 14, both the metastable α-Cu4Ti phase and the stable β-Cu4Ti phase were clearly and simultaneously observed. This difference is presumably attributable to the higher concentration of Ti in Cu–8 mass% Ti. Therefore, from the results shown in Figs. 14 and 15, it appears that a higher annealing temperature and an increased Ti concentration are the driving forces for forming both the metastable α-Cu4Ti phase and the stable β-Cu4Ti phase.
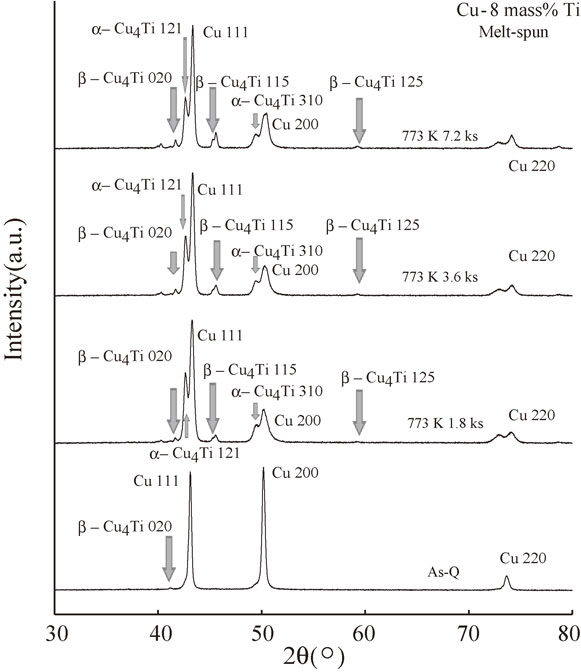
XRD spectra of the melt-spun Cu–8 mass% Ti alloy samples aged at 773 K for 0 (As-Q), 1.8, 3.6, and 7.2 ks.
We investigated the solid solubility extension in melt-spun Cu–Ti alloys using a combination of XRD and TEM. The results revealed that the solid solubility extension of Ti in the Cu matrix is in the range of 7–8 mass% Ti, whereas the solid solubility was less than 6 mass% Ti for samples fabricated using the conventional method (quenched samples). The difference in solid solubility between the melt-spun and quenched samples is due to the different cooling rates. The extremely high cooling rate of approximately 8 × 105 K/s used for the melt-spun samples easily leads to more Ti being dissolved in the Cu matrix. However, as pointed out by Xi et al.38) and Mula et al.,40) a greater amount of Ti can be expected to dissolve in the Cu matrix with the use of MA.
Furthermore, based on the XRD, BF TEM, and SADP results, it was demonstrated that melt-spun Cu–6 and 7 mass% Ti alloys undergo spinodal decomposition upon aging at 673 K. In this study, however, the presence of superlattice reflections has not yet been investigated.
For the samples possessing a Ti content of 8 mass%, small amounts of the stable β-Cu4Ti and metastable α-Cu4Ti phases were detected upon rapid cooling. No growth of the stable β-Cu4Ti phase was observed, however, because the annealing temperature of 673 K and annealing time of up to 7.2 ks were insufficient for the growth of this phase, which is mainly formed at high temperatures exceeding 773 K. Actually, the stable β-Cu4Ti phase was easily detected at an aging temperature of 773 K. In contrast, the formation of the metastable α-Cu4Ti phase is presumably promoted during annealing at 673 K. However, both the stable and metastable phases were simultaneously detected after annealing at 773 K.