2019 Volume 60 Issue 3 Pages 400-404
2019 Volume 60 Issue 3 Pages 400-404
Perovskite is an inevitable phase formed in the cathode during electrolytic deoxidation using titanium dioxide as the raw material by FFC process. Here, titanium suboxides (Ti3O5, Ti2O3 and TiO) are used as the raw materials to study whether perovskite can be avoided in the process. The results show that there is not perovskite formed during electrolysis using TiO and perovskite is appeared in the cathode when Ti3O5 and Ti2O3 are used as the raw materials. Perovskite is formed by combining Ca2+ in the molten CaCl2 with the cathode materials after the voltage is applied between the electrodes. Perovskite is mainly produced by electrochemical deoxidation process and amount of it gradually decreases with oxygen content decreasing in the cathode.
Titanium has a wide range of applications as an excellent structural and functional material. At present, the Kroll route is the main method for the preparation of titanium metal in industry. However, it has problems such as high preparation cost, complex process and serious pollution. Therefore, researchers have been seeking new technologies for efficient production of titanium. Electrolysis in molten-salt is been considered having great development prospects and has attracted worldwide attention. In 2000, Chen et al.1) carried out the direct electrochemical reduction of titanium oxide to titanium in molten CaCl2, which named the FFC (Fray-Farthing-Chen) Cambridge Process. In the FFC process, the mechanism of electro-deoxidation is that TiO2 is electrolyzed into titanium and oxygen ions at the cathode, oxygen ions are dissolved in molten CaCl2 and subsequently transferred to the anode under the electrolytic field. Finally, these ions discharged at the graphite anode in the form of CO or CO2.2,3) In the OS process,4–7) Suzuki et al. believed that the removal of oxygen via electrochemistry can be attributed to the calcium thermal reduction. There are different explanations about the mechanism of producing titanium by electrolysis process in molten salt.
Perovskite is the inevitable phase formed in the cathode during electrolysis process of stepwise deoxidization of TiO2.8–11) The formation of CaTiO3 can causes expansion in the solid phase and decrease of cathode porosity, which will slows ion transport in the pores existing between the oxide particles and slow down the electrolysis speed.12,13) In the later stage of electrolysis, reduction of TiO to Ti being another kinetically slow step. For the kinetic barrier caused by perovskite, Chen et al. studied the deoxidization effect of samples with different porosity, and found that increasing the porosity of samples can effectively reduce this barrier.14) Therefore, one of the factors for improving the efficiency of electrolysis is to avoid the formation of perovskite as much as possible.
In all reported on electroreduction of solid oxides, such as TiO2,12) Nb2O5,15) ZrO2,16) Ta2O5,17) and SiO2,18) calcium-enriched oxide or perovskite phases of various compositions are observed in partially reduced cathodes. However, the mechanism of formation of these intermediate products not fully understood. There are different views on the formation mechanism of perovskites. Schwandt and Fray et al.19) believe that during the process of electro-deoxidation of titanium dioxide, the CaTiO3 is formed when titanium dioxide was reduced to low-oxide. However, some other researchers20) believe that part of molten salt CaCl2 can be hydrolyzed to form CaO, which reacts with TiO2 to produced CaTiO3. In this paper, the formation mechanism of perovskites is determined by electrolysis using titanium oxides as the raw materials.
In previous studies, the results of different experiments using TiO2 as raw materials show that perovskite is formed rapidly during the deoxidation of TiO2. Figure 1 shows the xrd patterns and SEM images of TiO2 after electrolysis for 10 minutes. It shows that TiO2 reduces to Ti4O7 in a short time, and at the same time, perovskite is rapidly generated.

(a) XRD patterns of TiO2 after electrolysis for 10 minute; (b) SEM image of TiO2 after electrolysis for 10 minute.
Is there perovskite formed in the cathode if titanium suboxides are used as the raw material. In this paper, the main aim is to confirm the formation mechanism of calcium titanate in the cathode and the relationship between perovskite formed and raw material used in cathode.
The cathode raw materials used in this research including Ti3O5, Ti2O3, TiO powder with an average size of 40 µm–50 µm, and was wrapped by stainless steel gauze (400 mesh) as cathode for electrolysis. Anhydrous CaCl2 of 96% specified purity, purchased from the Chengdu Hua Gong Company was the electrolyte. And the dense alumina crucible of 100 mm internal diameter and 100 mm height was placed in the vertical tubular reactor. The crucible contained approximately 600 g of anhydrous CaCl2 salt. The KI solution (100 ml 0.2 g/L soluble starch and 0.08 g/L KI) was prepared to detect Cl2 gas in the electrolysis process.
The Ar with purity of 99.999% was flow into the vertical tubular reactor during all these experiments, the flowrate of gas is 200 mL/min. #304 Stainless Steel rods, 75 cm length, were selected as the current conductors. The dense graphite rod with purity of 99.9% and a diameter of 10 mm was connected to the steel rod by threaded connection, which was selected as the anode.
The electrolysis process was performed in a tubular electrolytic furnace (show in Fig. 2). The stainless steel lid seals the upper end of the reactor, which was equipped with holes for the electrode rods and gas outlet. Argon gas continues to be introduced into the reactor from the bottom, and the gas exhaust was led into the starch KI solution to detect the released Cl2 gas.

Schematic of the apparatus.
Put the corundum crucible into electrolytic furnace, which contains anhydrous calcium chloride, and the furnace lid was sealed. Then, the sealed reactor system was vacuumed and constantly flushed with high-purity argon gas, after the air in the furnace was completely discharged, argon gas was continuously introduced into the furnace to ensure an inert atmosphere. The furnace was heated from room temperature to 900°C, heating rate was 10°C/min, and keep it for 30 minutes to ensure calcium chloride completely melted and the stability of the furnace temperature. After that, two stainless steel rods were inserted into molten CaCl2 as the electrode rods, a stainless steel bar with graphite bar at the bottom was used as anode, and another stainless steel rod as cathode. Then a voltage of 2.8 V was applied between the cathode and the anode for pre-electrolysis to remove impurities in molten salt.
The titanium suboxides powder (Ti3O5, Ti2O3 and TiO) was wrapped in stainless steel net as the cathode and graphite rod was used as the anode, a constant voltage of 3.1 V applied between the anode and the cathode. The electrochemical reduction was interrupted after different reaction times (10 minutes, 1 hourand 2 hours respectively), then the cathode was removed from the salt melt and locating it in the upper part of the reactor. The residual molten salt on the surface of the sample rapidly solidifies with decreasing temperature, and argon gas was kept in the furnace to ensure the inert atmosphere, these procedures can avoid increasing the oxygen content in sample during cooling. After cool down to room temperature naturally, put the samples into the water for several hours to remove the soluble residue CaCl2 from sample surface and then dried. Finally, the dried samples were used for the XRD and SEM analyses.
2.3 Characterization of the cathodic productsThe phase composition of the samples was determined through X-ray diffraction analysis (XRD: D/max 2500PC). Each scan was 10° to 90°. The microstructure and chemical composition of the samples were investigated using scanning electron microscopy (SEM, TESCAN VEGA II) and energy-dispersive X-ray analysis (EDS, Oxford INCA Energy 350). The acceleration voltage was 15 eK.
Figure 3(a) shows that Ti3O5 powder used as the cathode in the electrolysis process and XRD patterns.

Ti3O5 used as the cathode (a) the XRD patterns of Ti3O5 powder at different electrolytic time; (b) Changes of Starch KI solutions.
According to the XRD patterns, it shows perovskites formed after electrolysis for 10 minutes and Ti3O5 completely reduced to Ti2O3. With the prolongation of the reduction time, the Ti2O3 is reduced to Ti2O after 1 hour and it is further reduced to Ti3O after 2 hours. Perovskite phase also appeared in the XRD pattern of the sample after electrolysis in 10 minutes and 2 hours. This is due to a large amount of Ca2+ from the molten CaCl2 entered the cathode after the voltage was applied between the electrodes, these Ti3O5 are then reduced to Ti2O3, with the perovskite produced as a byproduct.
When Ca2+ from the molten salt takes part in the reaction to formed perovskite in the cathode, Cl− may be forced to accumulate at the anode and finally be released as Cl2. The chlorine reacted with KI in the solution and replaced the iodine, so the replaced iodine produced a colour reaction with the starch in the solution. Figure 3(b) shows that the solution turns blue after electrolysis, which proves the formation of perovskite during electrolysis.
3.1.2 Electrolysis of Ti2O3In the same way, the Ti2O3 powder as the cathode material, the phase compositions of partially reduced samples at different electrolysis time show in Fig. 4(a).

Ti2O3 used as the cathode (a) the XRD patterns of Ti2O3 powder at different electrolytic time; (b) Change of Starch KI solutions.
After the voltage was applied between the anode and cathode, the Ti2O3 is rapidly reduced to TiO in 10 minutes, and it is also accompanied by the formation of perovskite as the byproduct. CaTi2O4 can be found in the sample after electrolysis for 1 hour, which may be caused by chemical reaction occur between TiO and CaTiO3, and a part of the CaTi2O4 is deoxidized to form Ti2O. In the XRD patterns electrolysis for 2 hour, it show Ti2O was deoxygenated to produced Ti3O, and there was no CaTiO3 and CaTi2O4 in the sample. In Fig. 4(b), the phenomenon of bluing of starch KI solutions also proves the production of perovskite in the process of electrolysis.
3.1.3 Electrolysis of TiOThe result show that there is no CaTiO3 formed if TiO is used as the raw materials. Figure 5 shows the XRD patterns and the change of the gases released from the anode. In Fig. 5(b), the starch KI solutions also did not detect chlorine gas. Part of TiO has been reduced to Ti3O after 10 minutes. It was completely reduced to Ti3O in 1 hour, Ti6O was produced after 2 hours.

TiO used as the cathode (a) the XRD patterns of TiO powder at different electrolytic time; (b) Change of Starch KI solutions.
The results show that perovskite formed in the cathode can be avoided only by using TiO as the raw materials during the electrolysis. Perovskite also appeared in the cathode when Ti3O5 and Ti2O3 are used as the raw materials. Comparing the results of Fig. 3 with Fig. 4, CaTiO3 is still detected in the sample after electrolysis for 1 hour using Ti3O5. However CaTiO3 is completely converted to CaTi2O4 when using Ti2O3. This means that using Ti2O3 as the raw materials produces less perovskite than that of Ti3O5. The results demonstrate that as the oxygen content in the cathode raw material decreases, the amount of perovskite gradually reduced and there is not perovskite formed during electrolysis TiO.
We all know that CaCl2 is easly hydrolysis in air to produce CaO. CaO can be react with TiO2 formed CaTiO3. To prove that whether CaTiO3 in the cathode produces during cooling, washing or drying in air after electrolysis. The comparison experiments of with voltage and without voltage are proceeded at here.
The results in Fig. 6 show that no perovskite produced when the sample soaks in the molten CaCl2 compared with the sample electrolysized in molten CaCl2 at the same condition. It proved that perovskite in the cathode is only formed during electrolysis.
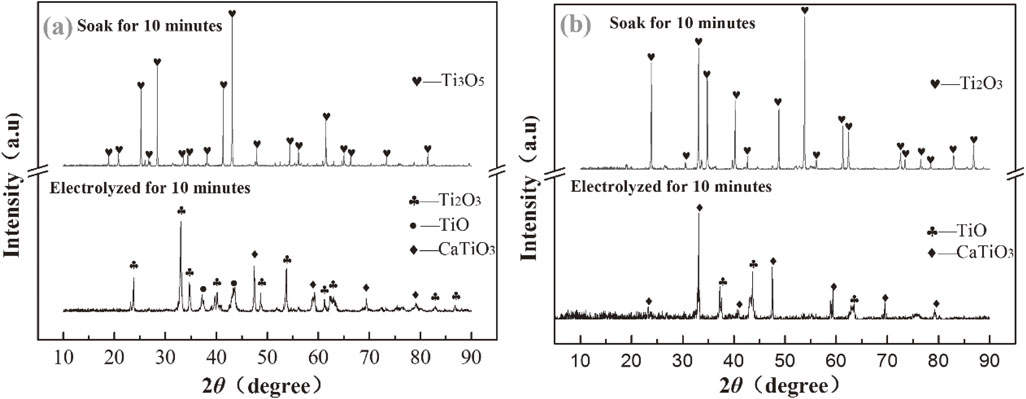
(a) Comparisons of the XRD patterns of Ti3O5 powder electrolysis for 10 minutes and soak for 10 minutes; (b) Comparisons of the XRD patterns of Ti2O3 powder electrolysis for 10 minutes and soak for 10 minutes.
In order to investigate the relationship between the changes of themorphology and the reaction of the samples during the deoxidization process, the partially reduced sample was measured by scanning electron microscope.
SEM image in Fig. 7(a) shows that there is a large number of perovskite electrolysis for 10 min using Ti3O5, which exhibit a crystal-like morphology and densely aligned with each other. In the initial stage of electrolysis, Ti3O5 reduces to Ti2O3, the perovskite grows rapidly with terraced structure. Schwandt21) believes that this terracing results from the CaTiO3 “attempting” to form a uniform layer around the irregularly shaped sub-oxide particles, while maintaining faceted growth and one crystallographic orientation. It can be observed that the edge of the crystal structure becomes slippery gradually in 1 h shown as in Fig. 7(a). The change of microstructure is due to the chemical reaction between TiO and CaTiO3 formed CaTi2O4 (reaction 3). CaTiO3 or CaTi2O4 was not found in the the sample electrolysis for 2 hours. It is consistent with the XRD results. Figure 7(b) is the SEM images using Ti2O3 electrolysis at different times. It can be found that the amount of perovskite produced is much when using Ti3O5 as the raw metarial in 10 min. Namely, oxygen content in titanium oxides is higher amount of perovskites is more. This is consistent with the dissolution behavior expected for a faceted material, which will grow by a ledge mechanism and preferentially dissolve at the corners.21) It can be inferred that the stage of perovskite formation has ended and some perovskites have begun to react with TiO to form CaTi2O4. It is obvious that the amount of perovskite produced decreases with the decreasing of oxygen content in the raw material and the reaction proceeds to the next stage faster.

SEM images using different titanium suboxides electrolysis at different time (a) using Ti3O5 as the raw material electrolysis for 10 min, 60 min and 120 min (b) using Ti2O3 as the raw material electrolysis for 10 min, 60 min and 120 min; (c) using TiO as the raw material electrolysis for 10 min, 60 min and 120 min.
The crystal-like morphology particles not appeared in Fig. 7(c). It is consistent with the results of the XRD pattern. The microstructure shows that the structure of samples becomes denser with the decrease of oxygen content.
The authors gratefully acknowledge gratefully the financial support from the National Natural Science Foundation of China (Grant No. 51674054), supported by the National Key R&D Program of China (2017YFB0603801), and supported by the Chongqing Key Laboratory of Vanadium-Titanium Metallurgy and New Materials, Chongqing University, Chongqing 400044, PR China.