2019 Volume 60 Issue 3 Pages 458-463
2019 Volume 60 Issue 3 Pages 458-463
The adsorption capacity of rice hull charcoal for cesium and strontium in aqueous solution was examined. Rice husk carbonized at various temperatures was added to aqueous solutions of cesium and strontium and stirred, whereupon, samples carbonized at 400°C adsorbed cesium, and those carbonized at 800°C adsorbed strontium. In view of this result, it was inferred that the mechanisms of adsorption of cesium and strontium on rice hull charcoal are different. FTIR analysis and Boehm titration results indicated that charcoal carbonized at 400°C had acidic functional groups. In addition, the pH of the rice hull charcoal carbonized at high temperature tended to be higher, which is attributed to precipitation of inorganic substances such as potassium on the surface of rice hull charcoal. Pore distribution measurements showed that many mesopores were formed in rice hull charcoal carbonized at 800°C. It was concluded that cesium adsorbs primarily on acidic functional groups on the surface of rice hull charcoal and strontium undergoes ion exchange with potassium, or adsorbs on mesopores.
This Paper was Originally Published in Japanese in J. Soc. Mater. Sci., Japan 67 (2018) 898–903.
Following the accident at Fukushima Daiichi Nuclear Power Station due to the Great East Japan Earthquake that occurred in March 2011, many radioactive substances such as cesium and strontium were released, as a result of which, water, soil, and air were contaminated. It is a matter of great urgency to remove these radioactive materials; for this purpose, zeolite is currently being used as the adsorbent material for radioactive substances present in water and soil. However, zeolites show high selectivity in their adsorption behavior, which means that many different types of zeolites are necessary for total decontamination. Moreover, there are problems with regard to zeolite reserves and also concerning disposal methods for the same.1)
The concentration of 137Cs in contaminated water collected at the Central Waste Treatment Building (Process Main Building) at Fukushima Daiichi Nuclear Power Station prior to processing with a cesium removal apparatus varies depending on when the sample was collected. For example, this concentration varies from 1.8 × 109 Bq/L (2011 July 28) to 1.1 × 108 Bq/L (June 19, 2012). The amount of contaminated water generated from the reactor building, as declared on July 28, 2011, was 398 kL/day and that on June 19, 2012 was 540 kL/day.2,3)
In the nuclear reactor building waste water of Unit 3 of the Three Mile Island Nuclear Power Station in the United States (July 1, 1980), the concentrations of 137Cs were reported to be 160 µCi/mL (5.9 × 109 Bq/L); 134Cs, 26 µCi/mL (9.6 × 108 Bq/L); 90Sr, 2.3 µCi/mL (8.5 × 107 Bq/L); 89Sr, 0.53 µCi/mL (2 × 107 Bq/L); and Cs, 0.8 ppm and strontium, 0.1 ppm.4) There is also a report that the relationship between the Bq unit of 137Cs and ppm concentration corresponds to 3.2 × 109 Bq/L of 1 ppm.5)
The adsorption of cesium by wood resources such as charcoal has also been considered to be an effective strategy to decontaminate water containing Cs. Charcoal has a high adsorption capacity for various substances, has little adverse effect on the ecosystem, and is ubiquitous due to the large natural abundance of wood that allows its mass production. In addition, charcoal is stable and not easily decomposed for long periods of time.6)
Lumber from thinning and bamboo can be considered as source materials for charcoal. There are however, a limited number of reports on the adsorption of cesium by charcoal. Ershov et al.7) showed that cesium in water can be adsorbed on charcoal due to the presence of acidic functional groups such as phenolic groups present in the micropores of charcoal. Moreover, Yamauchi et al. reported that charcoal had adsorption capacity for cesium8,9) and Itoh et al. reported that bamboo charcoal had adsorptivity towards cesium, strontium, and iodine.10,11)
In Japan, about 8 million tons of rice is harvested annually, of which about one fifth constitutes chaff. For the treatment of rice hulls, open burning has traditionally been performed; however, in recent years, this process has faced stricter regulations due to problems such as air pollution and malodor, and consequently, most rice hulls are treated as waste without being utilized. It is also known that rice husk coal can be used in deodorization, water purification, and soil conditioning. Furthermore, the adsorption performance of rice hull for aldehyde gas has been reported.12,13) Shozugawa et al. have reported that rice hulls can adsorb radioactive cesium.14) However, studies on the cesium and strontium adsorption of rice hull charcoal have not been conducted so far. In this study, we investigated the possibility of effectively adsorbing cesium and strontium present in aqueous solutions, on rice hull coal.
Dried rice husk (10.0 g, Harebare, manufactured by Aichi Prefecture 2015) was placed in a capped 100 mL alumina crucible, and heated to different temperatures with a heating rate of 18.0°C/min using a separator type desk electric furnace (AMF 20-D, Hitachi High-Technologies Corporation). After reaching the set temperature, the samples were maintained at that temperature for 1 h to carbonize them.15) The set temperatures were 400°C, 600°C, 800°C, and 1000°C.
The yield Y (%) of rice hull charcoal carbonized at each temperature was calculated according to the following equation.16,17)
| \begin{equation} \mathrm{Y} = (\text{W$_{0}$}/\text{W$_{\text{L}}$})\times 100 \end{equation} | (1) |
Where W0 and WL are the dry masses of rice hull charcoal after and before carbonization, respectively.
2.2 Characterization of rice hull charcoalThe morphology of the rice hull charcoal produced was observed by scanning electron microscopy (SEM, S-2600N, Hitachi High-Technologies). Thermal analysis was performed using a differential thermo balance (Thermo plus TG 8120, Rigaku Corporation).
In accordance with the standard procedure JIS K 1474, 100 mL of water was added to 1.00 g of a powder sample and the mixture was heated for 5 min so as to boil it. The solution was then cooled to room temperature and made up to 100 mL by adding water and stirred thoroughly, after which the pH of the suspension was measured using a pH meter (SevenMulti METTLER TOLEDO).
The specific surface area of the rice hull charcoal sample was measured from adsorption-desorption isotherms by the BET method (Monosorb Yuasa Ionics Co., Ltd.). The functional groups present on the charcoal sample were identified by Fourier transform infrared (FT-IR, FT-IR-680 Plus JASCO) spectroscopy. In addition, the Boehm method18,19) was used to measure the total amount of acidic functional groups present on the surface of the rice hull charcoal sample. Rice husk coal (0.10 g) and 30 mL of a 0.10 mol/L NaOH aqueous solution were added to an Erlenmeyer flask and shaken at 25°C for 5 days. The mixture was then filtered and the filtrate back-titrated with an aqueous HCl solution of 0.10 mol/L, from which, the amount of acidic functional groups was measured from the volume of HCl consumed.
The pore size distribution of rice husk coal was measured using a pore distribution measuring device (Autosorb-1 Quantachrome).
2.3 Adsorption experimentAqueous solutions of cesium chloride and strontium chloride were prepared so as to have concentrations of the respective metal elements of 10 ppm, 50 ppm, and 100 ppm. To each solution, 1 g of rice husk charcoal pulverized to 250 µm size or less, was added and stirred for 1 h. The solution was then filtered and the concentration of the metal element in the filtrate was measured using a polarized Zeeman atomic absorption photometer (Z-2300, Hitachi High-Technologies Corporation).
2.4 Potassium dissolution experimentTo 100 mL of water, 1 g of rice husk charcoal pulverized to 250 µm or less, was added and stirred for 1 h. The solution was filtered and the concentration of potassium in the filtrate was measured using a polarized Zeeman atomic absorption spectrophotometer (Z-2300, Hitachi High-Technologies Corporation).
Figure 1 shows the relationship between the carbonization temperature and the yield of rice husk coal. The yield of rice hull charcoal carbonized at 400°C was 47.5%, but for rice husk charcoal carbonized at 1000°C, the yield decreased to 23.0%. Figure 2 shows the results of thermal analysis of rice hulls. A weight loss around 100°C, as well as a large exothermic reaction around 300 to 400°C accompanied by further weight loss, were seen.

Yield of rice hull charcoal at different carbonization temperatures.

Thermal analysis of rice husk.
It is thought that the weight loss around 100°C is due to the evaporation of moisture in chaff and that in the temperature range 300–400°C can be attributed to the combustion of organic matter such as cellulose and lignin.
3.1.2 Specific surface area of rice hull charcoalFigure 3 shows the SEM image of rice hull charcoal. Kawamura et al.20) reported that carbonizing at 500°C in a stream of nitrogen burns the cuticle layer, but the carbonized wood layer and the silica layer remain closely bonded to each other to create a robust macro-shell and a micro-shell structure.

Rice hull charcoal for different carbonization temperatures. (a) 400°C, (b) 600°C, (c) 800°C, (d) 1000°C.
In this study as well, as shown in Fig. 3, it is observed that the macro-shell and the micro-shell structure are retained even after carbonization. In addition, a part of the carbonized woody layer is observed to be porous; with increase in carbonization temperature, the wall thickness tends to decrease slightly.21,22) Figure 4 shows the relationship between the carbonization temperature and the specific surface area of the rice husk coal. An increase in specific surface area is observed for higher carbonization temperatures. However, the specific surface area of rice hull charcoal carbonized at 1000°C shows a decreasing trend.
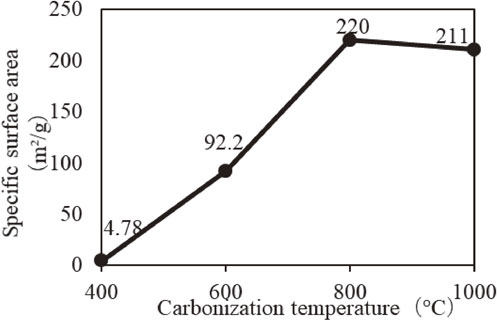
Specific surface area of rice hull charcoal carbonized at different temperatures.
The increase in specific surface area of rice hull charcoal carbonized at high temperature is believed to be due to the burning of organic matter contained in the rice husk, which generates pores by carbonization. Moreover, a large number of tiny pores, too small to be observed by SEM, are generated. The decrease in the specific surface area for carbonization at 1000°C is attributed to the reaction between silica and potassium contained in the rice hull to form compounds such as potassium polysilicate, which led to closed pores.23) In addition, there is a possibility that the pores become smaller due to thermal shrinkage.
3.1.3 pH of rice hull charcoalTable 1 shows the relationship between the carbonization temperature and the pH of rice husk coal. It is seen that the pH tends to increase with carbonization temperature.

Previous reports have concluded that a high carbonization temperature causes the precipitation of inorganic substances such as potassium contained in the epidermis of rice husk on the surface of rice husk; these are then easily eluted, which increases the pH.13)
3.1.4 Measurement of acidic functional groups on rice hull charcoalFigure 5 shows the FT-IR spectra of rice husk coal heated at 400°C and 600°C. Rice hull charcoal shows absorption at 1700 cm−1 which is attributed to C=O stretching vibration and the absorption at 3300 cm−1 is considered to be due to O–H stretching vibration. For rice hull charcoal carbonized at 800°C and 1000°C, these absorption peaks weakened and nearly disappeared. From these results, it is suggested that in rice hull charcoal carbonized at 400 and 600°C, acidic functional groups such as carboxyl group and phenolic hydroxyl groups are present. Table 2 shows the Boehm titration results to determine the concentration of acidic functional groups present on the rich hull charcoal. Similarly, acidic functional groups were found in rice hull charcoal samples carbonized at 400°C and 600°C, but the presence of these groups could not be confirmed for samples carbonized at 800°C and 1000°C. It has been reported that the concentration of acidic functional groups such as carboxyl and phenolic hydroxyl groups decreased when heated above 580°C.24,25) Hence, we believe that the presence of these groups cannot be confirmed for rice husk coal samples carbonized at high temperatures.

FTIR spectra of rice hull charcoal carbonized at different temperatures.

Figure 6 shows the measured data for the pore distribution for rice husk coal samples carbonized at different temperatures. For the sample carbonized at 400°C, due to the incomplete burning of organic matter, the pore volume remained small since no pores were formed. For rice hull charcoal carbonized at temperatures >600°C, pores were formed, and in particular, the development of pores of diameters in the 2 to 50 nm range, also known as mesopores, was confirmed.
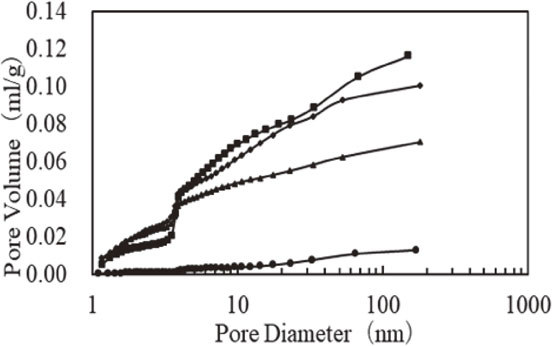
Pore size distribution of rice hull charcoal carbonized at different temperatures.
According to the experimental method described in “Fundamental data for contaminated water treatment technology in Fukushima Daiichi Nuclear Power Station” in the database of the Division of Nuclear Fuel Cycle and Environment (NUCE) Atomic Energy Society of Japan (AESJ), a concentration in solution of ∼1 to 10 ppm for cesium or strontium is used to measure the rates of adsorption of these metals rates on various materials.26)
In this study, with reference to the standard technique for nuclear reactor-contaminated water as described above, we have also used cesium or strontium solution of concentration in the 10 to 100 ppm range for our adsorption experiment.
3.2.1 Adsorption of cesiumFigure 7 shows the results of adsorption cesium present in an aqueous solution on rice hull charcoal. For all concentrations of the cesium solution, the adsorption rate of cesium tended to be higher for rice hull charcoal with a lower carbonization temperature. Rice hull charcoal carbonized at 400°C adsorbed 82.8% cesium in 10 ppm cesium solution, 75.4% in a 50 ppm solution, and 64.5% in a 100 ppm solution.

Adsorption rates of cesium and strontium from solutions of different concentrations (a) 10 ppm, (b) 50 ppm, (c) 100 ppm.
It can be assumed that the adsorption of cesium on rice hull charcoal is mainly due to physical adsorption in the pores and the like, and chemisorption on acidic functional groups and the like.2,27) It has been reported that acidic functional groups effective for cesium adsorption decrease when heated above 580°C.24,25) As shown in Fig. 5 and Table 2, rice hull charcoal carbonized at 400°C underwent C=O stretching vibration and O–H stretching vibration, which could be attributed to acidic functional groups. However, rice hull charcoal carbonized at 1000°C did not show peaks due to these stretching vibrations. From these results, we conclude that carbonizing at high temperature decreased the amount of acidic functional groups and thereby decreased the cesium adsorption ratio. However, for rice hull charcoal with carbonization temperatures of 800°C or higher, a greater development of porosity was observed (Fig. 6), but the presence of acidic functional groups could not be confirmed. Thus, the adsorption of cesium by physical adsorption to pores was observed.
3.2.2 Adsorption of strontiumRice hull charcoal carbonized at 600°C or higher temperatures, adsorbed 99% or more of strontium in a 10 ppm strontium solution. Samples carbonized at 800°C adsorbed 97.4% in a 50 ppm strontium solution and 80% strontium in a 100 ppm solution. The adsorption rate of strontium tended to increase with increase in carbonization temperature, but decreased slightly for the rice hull charcoal carbonized at 1000°C. Strontium adsorption to rice hull charcoal is believed to be due to adsorption in pores and to cation exchange with potassium in ash. It has been reported that mesopores present in rice hull charcoal are effective for the adsorption of cations such as strontium.27) From the results of the pore distribution shown in Fig. 6, it can be seen that rice hull charcoal with a high carbonization temperature contains mesopores. Figure 8 shows the relationship between mesopore volume and the strontium adsorption rate. This graph shows that the mesopore volume and strontium adsorption rate are highly correlated with R = 0.939.

Relation between strontium adsorption rate and mesopore volume.
Moreover, to verify if the strontium is cation-exchanged with potassium, the amount of potassium eluted from the rice husk charcoal in water was measured using an atomic absorption photometer; the results are shown in Fig. 9. The eluted amount of potassium increases with carbonization temperature but decreases slightly for the sample carbonized at 1000°C.

Amount of eluted potassium in rice hull charcoal carbonized at different temperatures.
Figure 10 shows the relationship between the eluted amount of potassium and the strontium adsorption rate. A high correlation coefficient of R = 0.961 shows that strontium adsorption rate is highly correlated with eluted potassium amount.

Relation between strontium adsorption rate and volume of potassium eluted.
The capacity of adsorption by rice husk charcoal, of cesium and strontium present in aqueous solution was examined.
I am grateful to Kinjo Gakuin, University Faculty of Pharmacy, for cooperation in the experiment and in preparing this paper.