2019 Volume 60 Issue 4 Pages 587-592
2019 Volume 60 Issue 4 Pages 587-592
A new process of internal activation of carbon fiber reinforced thermoplastic polymer (CFRTP) of polypropylene (PP) by applying electron beam irradiation (EBI) under oxygen (O2)–rich nitrogen gas (N2) atmosphere to CF chopped strand matt (CSM) layers prior to assembly and hot press to strengthen the typically weak CF/thermoplastic polymers (TPs) adhesion was proposed. Samples were interlayered composite with layup of alternating PP and CF plies, [PP]4[CF]3. Composite fabrication was performed by one directional hot-press under constant pressure of 4.0 MPa at 473 K for 1 min. Results showed applying an optimum 0.22 MGy-EBI under protective N2 gas with O2 concentrations between 200 ppm and 200,000 ppm mostly improved the bending strength (σb) while reducing strain at the bending strength (σb) apparently increasing the elasticity. The method appears to work well for the weakest samples in the data sets: at low accumulative probability Pf = 0.06 by median rank method, σb was apparently improved by the 200 ppm and 2,000 ppm O2 atmospheres. Namely, 0.22 MGy-EBI under N2 gas atmosphere with 200 ppm to 2,000 ppm-O2 improved σb at Pf = 0.06 (57 MPa) about 21%, over that of untreated (47 MPa). Strength increase could be explained by mutual entangling of both sizing epoxy film on CF and PP with strong covalent bonding, which formation of direct  induced by EBI and oxygen assisted
induced by EBI and oxygen assisted  by concentrating the O2 gas molecules from 200 ppm to 2,000 ppm-O2 in N2 atmosphere, rather than weak molecular bonding CF-(H2O, N2, O2)-PP for the untreated samples. Moreover, the action of the EBI apparently acts to clean residual H2O, N2, and O2 to purify and activate the CF surface increasing polar group and active site density. They most likely contributed to bending strength enhancement. The 0.22 MGy-EBI in O2-rich N2 atmosphere appears to be a viable method to increase carbon fiber-thermoplastic polypropylene adhesion enhancing reliability and safety of the PP-CFRTP.
by concentrating the O2 gas molecules from 200 ppm to 2,000 ppm-O2 in N2 atmosphere, rather than weak molecular bonding CF-(H2O, N2, O2)-PP for the untreated samples. Moreover, the action of the EBI apparently acts to clean residual H2O, N2, and O2 to purify and activate the CF surface increasing polar group and active site density. They most likely contributed to bending strength enhancement. The 0.22 MGy-EBI in O2-rich N2 atmosphere appears to be a viable method to increase carbon fiber-thermoplastic polypropylene adhesion enhancing reliability and safety of the PP-CFRTP.
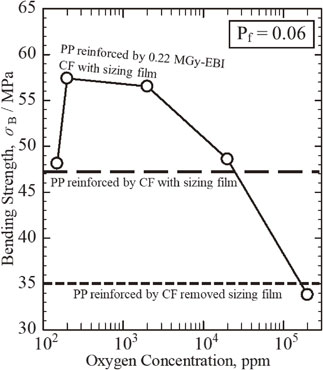
Fig. 6 Oxygen concentration dependent bending strength (Pf = 0.06) of PP reinforced by CF treated with 0.22 MGy-EBI under protective N2 gas atmosphere with each O2 concentration (solid lines), together with CF untreated with (thick broken line) and without (thin broken line) sizing film.
Climate change has been a serious problem for our earth. Therefore, lighter and stronger materials contributing to lowering vehicle CO2 emissions are essential. There is vital need for technical innovations such as lighter weight cars and development of electric vehicles (EVs) and solar vehicles (SVs) along with electric powered airplanes proposed by JAXA. Demand for carbon fiber reinforced polymers (CFRPs) has been expected to increase to replace high-tension steel components along with use for moldable EVs car bodies.
It follows thermoset polymers, widely used for CFRP such as epoxy have higher ultimate strength than thermoplastics (TPs). However, disadvantage is requiring long solidification times and cannot be recycled making mass-waste disposal a serious problem.
On the other hand, thermoplastic polypropylene (PP) is widely used as an eco-friendly commercial polymer that can be recycled and reformed, has shorter solidification cycle times, decent strength, and lower cost. Solidification time for PP is only about 10% that of epoxy reducing energy consumption. Other advantages of PP are heat resistance and high chemical resistance: while thermoplastics in general have higher crack resistance than thermosets. PP belongs to the polyolefin group being partially crystalline and non-polar.
However, the typically weak bonding between CF and TP pose serious challenges since CF lattice structure has graphitic basal planes with non-polar surface and is reported1–3) to be chemically inert due to manufacturing step of high temperature carbonization and graphitization.4) Moreover, surface smoothness, negligible adsorption characteristics, and lipophobicity lead to insufficient bonding with matrix materials.5,6) CF has drawback of poor adhesion with PP resulting in lower mechanical properties from easy fiber pull-out due to low contact area at non-polar CF/PP interface.
Therefore, increasing adhesion of CF to matrix material has been a major goal in composite design. The literature reports several CF surface treatment methods.1,2,7–20) Acidic modification creates a rougher surface possibly creating higher friction at the interface.7) However, it can cause surface damage and weight loss9) decreasing strength.2,8) Widely researched is plasma CF surface modifications10–12) reported to be successful in increasing interlaminar shear strength of CFRP. Plasma oxidation of CF was found to increase interfacial shear stress of CFRP epoxy composites from 6 MPa to 42 MPa.13)
Many methods to increase adhesion create polar functional groups on the CF surface.14–16) Electro-polymer coating has been carried out depositing polymer coatings on CF surface by chemical grafting reactions introducing functional groups –OH, –NH2, and –COOH to increase CF adhesion to epoxy, and the strength of CF itself.14) Rare earth particle attachment method increases functional polar groups on CF surface has improved mechanical properties.15,16)
Applying high energy irradiation to CF for example ions and γ-rays have been found to enhance fiber/matrix adhesion without use of catalyst.17,18) The high energy creates active sites in the crystal lattice while increasing surface roughness. High energy Ar+ ion irradiation (0.6 keV to 1.4 keV) showed carbonyl peak and broadened-OH peak of FTIR (Fourier-transformation infrared spectroscopy) increasing polarity and H-bond formation at CF interface.19) A 0.30 MGy dose of Co60 γ-ray irradiation has been reported to increase surface roughness of CF.20)
However, recently surface activation by applying a light electron (e−) charge by electron beam irradiation (EBI) to CF surface to increase adhesion with polymer matrix has had success. EBI is a relatively easy method that does not use atoms or any catalyst. Moreover, EBI does not require chemical treatment of the CF. Large areas, such as LED TV screens can be treated.
When EBI activates the CF surface, it decreases dangling bond density in the hexagonal graphite structure as evidenced by a decrease in electron spin resonance (ESR) peak intensity.21) CF has been reported to naturally contain dangling bonds in its hexagonal structure.21) The EBI has been reported to enhance crack resistance and increase tensile fracture stress and elasticity, increasing ductility strengthening the CF itself.22)
Moreover, EBI activated CF has been reported to increase adhesion to thermoplastics increasing tensile properties in [Ti/EBCF/ABS] joints 2.1 times higher than untreated [Ti/CF/ABS];23) and [Ti/EBCF/PC] joints 3.0 times higher than untreated [Ti/CF/PC].1) Increased charge site distribution at the CF surface is homogeneous since CF is a strong conductor of electricity. The increased charge at the CF surface contributes to bonding of CF with TPs. Since EBI appears to enhance the nucleation sites and then increases the friction force at CF/TP polymer interface, it prevents CF pull-out prior to dipping into thermoplastic ABS or PC, resulting in improving tensile strength.
Illustrated in Fig. 1 is schematic drawing of PP/CF interface for CFRP. Figure 1(a) shows the interface with air molecules from the atmosphere (O2, H2O(g), and N2) that are assumed to act to create weak Van Der Walls attractive forces for PP reinforced carbon fiber untreated. This results in typical low friction with low point contact density.

Schematic diagram of CF/PP interface untreated (a), and EBI-treated in O2-rich N2 atmosphere creating stronger covalent bonds (b).
Up to now, EBI in typical N2 atmosphere has been used to create stronger adhesion of CF to TPs1,23) with EBI acting to clean the CF surfaces while activating CFs increasing intermolecular bonding with CF hexagonal structure π electrons. However, to increase adhesion further, we employ the novel process of coupling the EBI activation with creating polar groups on the CF surface to proliferate covalent bonds as shown in Fig. 1(b). Since we found 0.22 MGy to be the optimum EBI dose for the [PP]4[CF]3 composite samples, we applied it in O2-rich N2 atmosphere for increased interfacial bonding and bending properties.
The purpose of the present study is to improve adhesion of carbon fiber to thermoplastic polypropylene by using a new process of applying EBI under oxygen (O2)–rich nitrogen gas (N2) atmosphere to interlayered carbon fiber chopped strand matts (CF-CSMs).
The 55 vol%-carbon fiber reinforced thermoplastic polypropylene (PP-CFRTP) samples were constructed with 3 layers of carbon fiber (300 g/mm3-Chopped strand matt (CSM), TR3110M, Mitsubishi Rayon Ltd. Tokyo) alternating between 4 layers of thermoplastic polymer sheet (Polypropylene; BC06C Novatec, Nissho Ltd. Tokyo).24) EBI (see next section) was performed on both sides of each CF-CSM prior to assembly into the [PP-CF-PP-CF-PP-CF-PP] layup represented here as [PP]4[CF]3.
After assembly, solidification was performed by one directional hot-press (IMC-185A, Imoto Machinery Co., Ltd.) under 4.0 MPa at 473 K for 1 min. Sample dimensions: length, width and thickness were 80 mm × 10 mm × 2 mm, respectively.
2.2 Condition of EBIThe CSMs were homogeneously irradiated by an electron-curtain processor (Type CB175/15/180L, Energy Science Inc., Woburn, MA, Iwasaki Electric Group Co., Ltd., Tokyo) prior to assembly with PP and hot press.25–34) The CFs were homogeneously irradiated by the linear electron beam gun with low energy through a titanium thin film window attached to a 240 mm diameter vacuum chamber. A tungsten filament in a vacuum was used to generate the electron beam at a low energy condition, where acceleration potential (VAcc: keV) of 170 keV and the irradiating current density (I) of 0.089 A × m−2.
Although the electron beam is generated in a vacuum, the irradiated samples are kept under protective nitrogen at atmospheric pressure. The distance between the sample position and the window was 25 mm. To prevent oxidation, the samples were kept in the protective atmosphere of nitrogen gas with a residual concentration of oxygen below 300 ppm. The constant flow rate of nitrogen gas was set to be 1.5 L/s at 0.1 MPa nitrogen gas pressure. Each irradiation dose (0.0432 MGy) was applied for only a short time (0.23 s) to avoid excessive heating of the sample; the temperature of the sample surface remained below 323 K just after irradiation. The sample in the aluminum plate holder (0.15 m × 0.15 m) was transported on a conveyor at a constant speed of 10 m/min. The sheet EBI was applied intermittently; one sweep going one way is 0.0432 MGy. Repeated irradiations to both side surfaces of the samples were used to increase the total irradiation dose. The interval condition of 30 s was applied between each sweep. The irradiated dosage was proportional to the irradiation current (I, mA) and number of irradiations (N), whereas it was inversely proportional to the conveyor speed (S, m/min).
The irradiation dose was controlled by the integrated irradiation time for each of the samples. Here, irradiation dose was corrected by using an FWT nylon dosimeter of RCD radiometer film (FWT-60-00: Far West Technology, Inc. 330-D South Kellogg Goleta, California 93117, USA) with an irradiation reader (FWT-92D: Far West Technology, Inc. 330-D South Kellogg Goleta, California 93117, USA). The dose was 0.0432 MGy at each irradiation. Based on the mean density (ρ: kg × m−3) and irradiation potential at the specimen surface (V: keV), the penetration depth (Dth: m) of EBI is expressed by the following equation.35)
| \begin{equation} D_{\text{th}} = 66.7\mathrm{V}^{5/3}/\rho \end{equation} | (1) |
| \begin{equation} V = 170\,\text{keV} - \Delta V_{\text{Ti}} - \Delta V_{\text{N2}} \end{equation} | (2) |
| \begin{align} \Delta V_{\text{Ti}} & = T_{\text{Ti}}/D_{\text{thTi}}\times 170\,\text{keV} = T_{\text{Ti${\rho}$Ti}}/[66.7\times (170\,\text{keV})^{2/3}]\\ & = (10^{-5}\,\text{m})\times (4540\,\text{kg$\,$m$^{-3}$})/[66.7\times (170\,\text{keV})^{2/3}] \\ &= 22.2\,\text{keV} \end{align} | (3) |
| \begin{align} \Delta V_{\text{N2}} & = T_{\text{N2}}/\mathrm{D}_{\text{thiN2}}\times V_{\text{Ti}} = T_{\text{N2${\rho}$N2}}/[66.7\times (V_{\text{Ti}})^{2/3}]\\ & = (25\times 10^{-3}\,\text{m})\times (1.13\,\text{kg$\,$m$^{-3}$})\\ &\quad /[66.7\times (170-22.2\,\text{keV})^{2/3}] = 15.2\,\text{keV} \end{align} | (4) |
| \begin{align} V & = 170\,\text{keV} - 22.2\,\text{keV} - 15.2\,\text{keV}\\ & = 132.6\,\text{keV} \end{align} | (5) |
Bending tests were carried out at room temperature using a testing method for 3-point bending test (IMADA Co., Ltd. DPU-50N/MX-500N/GA-10N).1) Here, distance between outside points on specimen, midpoint length, and head speed were 40 mm, 20 mm, and 10 mm/min, respectively. To evaluate the fundamental mechanical property, bending stress-strain curves were obtained by using crosshead displacement and confirmed by using video recording device.
Evaluating the accumulative probability of strength (Pf) is a convenient method of quantitatively analyzing experimental values and in industry, is often employed in statistical quality control (QC). Pf is expressed by the following equation which is a generalized form of the median-rank method.37)
| \begin{equation} P_{\text{f}} = (i - 0.3)/(N_{\text{s}} + 0.4) \end{equation} | (6) |
Figure 2 illustrates bending strength (σb) against accumulative probability (Pf) of the [PP]4[CF]3 of PP reinforced by active CF irradiated under protective N2 gas atmosphere with 150 ± 40 ppm-O2, together with untreated CF.

Bending strength (σb) against accumulated probability (Pf) of PP reinforced by active CF irradiated under protective N2 gas atmosphere with 150 ± 40 ppm-O2, with untreated CF.
EBI dose of 0.22 MGy under the N2 with 150 ± 40 ppm-O2 slightly improves σb below 0.2 of low Pf, as shown in Fig. 2. Since the most important practical σb for industrial application is the lowest value of Pf = 0.06, optimal 0.22 MGy-EBI dose under the N2 with 150 ± 40 ppm-O2 atmosphere, which is confirmed in Fig. 2 showing at the low-Pf = 0.06, is slightly larger than that of untreated. However, the caution is recommended since Fig. 2 shows higher doses greater than 0.30 MGy degrade σb below that of untreated. The maximum σb value is found at 0.22 MGy-EBI.
The σb difference from 0.94 to 0.06 of Pf (Δσb) can be defined to be one of indicators of reliability. The Δσb values are 42, 45, 48, 38 and 35 MPa for 0.04, 0.13, 0.22, 0.30 and 0.43 MGy, as well as 47 MPa for untreated. The EBI decreases the Δσb and it does not improve the reliability. The optimal irradiation dose to get the highest reliability is obtained from 0.04 MGy to 0.22 MGy.
3.2 Bending test of CFRTP of PP dependence of oxygen content in protective oxygenated nitrogen gas atmosphere during EBIFigure 3 shows the bending stress-strain curves at low accumulative probability (Pf) of 0.06 for the CFRTP samples by applying optimal condition of 0.22 MGy-EBI under protective N2 gas each 200 ppm and 2,000 ppm-O2 to the CF-CSM prior to assembly and hot press for the [PP]4[CF]3 samples. The maximum bending strength (σb) is improved about 21%, to 57 MPa over that of untreated (47 MPa) at low accumulative probability (Pf = 0.06). Moreover, bending elasticity (σ/ε)b has slightly increased.

Bending stress-strain curves at low accumulative probability (Pf) of 0.06 for the CFRTP samples at [0.22 MGy-EBI under protective N2 gas with 200 ppm and 2,000 ppm-O2] conditions, respectively.
Figure 4 shows the optical photographs of fractured samples (Pf = 0.06) of the PP reinforced by CF untreated (see Fig. 4(a)) and treated by 0.22 MGy-EBI dose under nitrogen gas atmosphere with 2,000 ppm oxygen concentration (see Fig. 4(b)). The photos show the treated sample in Fig. 4(b) has significantly less damage in the form of buckling, less ply separation in the form of peeling between the CF-CSMs and PP layers, and less fiber pullout than the untreated in Fig. 4(a).

Photographs fractured samples (Pf = 0.06) of PP reinforced by CF untreated (a) and treated by 0.22 MGy-EBI dose under 2,000 ppm-O2 rich nitrogen gas atmosphere (b).
Figure 5 illustrates the maximum σb (a) and strain εb (b) at σb for the EBI 0.22 MGy under protective N2 gas atmosphere as a function of O2 concentration.

Changes in bending strength (σb) against accumulative probability (Pf) (a) and its strain (εb) (b) for CFRTP of PP reinforced by 55 vol%-CF untreated and treated at 0.22 MGy-EBI dose under the O2-rich N2 atmosphere.
Figure 5(a) shows the 200 ppm O2 increased the σb at all Pf: from 76 MPa to 80 MPa; 58 MPa to 67 MPa; and 47 MPa to 57 MPa at high-, median-, and low-Pf of 0.94, 0.50 and 0.06, respectively. Moreover, Fig. 5(a) shows for the 0.22 MGy-EBI under N2 gas atmosphere with O2 concentrations from 200 ppm to 2,000 ppm increased the σb at all Pf less than or equal to medial 0.50.
Figure 5(a) also shows that the σb difference from 0.94 to 0.06 of Pf (Δσb), one of indicators of reliability are 23, 11, 18 and 40 MPa for 0.22 MGy under protective N2 gas atmosphere with 200 ppm, 2,000 ppm, 20,000 ppm and 200,000 ppm-O2, as well as 30 MPa for untreated and 12 MPa for 0.22 MGy EBI under N2 atmosphere with 150 ± 40 ppm-O2 (see Fig. 2). The 0.22 MGy-EBI under N2 gas atmosphere with optimal O2 concentration from 150 ± 40 ppm to 20,000 ppm apparently decreases the Δσb and improves the reliability.
On the other hand, Fig. 5(b) shows the 200 ppm O2 had apparent effect on the strain at σb (εb) at more than Pf = 0.6, although the εb is reduced acting to make the more ductile thermoplastic more brittle.
Figure 6 shows changes in bending strength (σb) at the lowest accumulative probability (Pf = 0.06) against O2-concetration in protective N2 atmosphere during 0.22 MGy-EBI of the weakest CFRTP samples of PP reinforced by 55 vol%-CF with sizing film treated (Solid line).

Oxygen concentration dependent bending strength (Pf = 0.06) of PP reinforced by CF treated with 0.22 MGy-EBI under protective N2 gas atmosphere with each O2 concentration (solid lines), together with CF untreated with (thick broken line) and without (thin broken line) sizing film.
The 0.22 MGy-EBI under N2 gas atmosphere with optimal O2 concentration from 150 ± 40 ppm to 200 ppm apparently increases the bending strength (σb) from 48 MPa to 57 MPa. Furthermore, the addition of O2 gas of more than 200 ppm decreases the σb from 57 MPa to 34 MPa.
Figure 6 also shows changes in bending strength (σb) at the lowest accumulative probability (Pf = 0.06) of the weakest CFRTP samples of PP reinforced by 55 vol%-CF with sizing film treated (solid line), together with untreated CF with and sizing film (thick broken and thin dotted lines),38) respectively. The covering sizing film has improved the strength from 35 MPa to 47 MPa.38) The improvement of fracture strength of CFRTP untreated is probably caused by the mutual entangling between sizing epoxy film and PP.
As shown in Fig. 6, the 0.22 MGy-EBI under N2 gas atmosphere with optimal O2 concentration from 200 ppm to 2,000 ppm apparently increases the bending strength (σb). It is probably explained by mutual entangling of both sizing epoxy film on CF and PP with strong chemical bonding.
The covalent bonding formation of direct $\text{CF:$\fbox{C:C}$:PP}$ induced by EBI and oxygen assists $\text{CF:$\fbox{C:O:C}$;PP}$ by concentrating the O2 gas molecules from 200 ppm to 2,000 ppm-O2 in N2 atmosphere (see Fig. 1(b)), rather than in air, molecular bonding CF-(H2O, N2, O2)-PP for the untreated samples (see Fig. 1(a)). Polar oxygen groups created on the CF surface most likely contributed to bending strength enhancement. Therefore, converting from weak molecular bonds to the strong covalent bonds and broadening the contact area at carbon fiber/thermoplastic polypropylene interface leads to higher resistance to fiber pull-out with large friction force.
Furthermore, the addition of O2 gas from 20,000 ppm to 200,000 ppm tremendously decreases the σb (34 MPa), which is below the σb (35 MPa) of CFRTP of PP matrix reinforced by untreated CF without sizing film. It can be explained that the EBI absorbs impurity atoms and damages the covalent bonds of sizing epoxy film, as well as graphite hexagonal structure of CF. Thus, it is difficult to generate the mutual entangling. In addition, the excess O2 proliferates weak $\text{F:$\fbox{C:O:H}$-(O}_{2}\text{)-$\fbox{H:O:C}$:PP}$ bonds (Fig. 1(a)), hence crowding the strong $\text{CF:$\fbox{C:C}$:PP}$ and $\text{CF:$\fbox{C:O:C}$;PP}$ bonds (Fig. 1(b)). Therefore, carefulness is highly recommended to adjust for optimum O2 concentration for practical applications.
Moreover, the action of the EBI apparently acts to clean residual H2O, N2, and O2 to purify and activate the CF surface increasing polar group and active site density. The three processes probably work simultaneously that composition ratio can be controlled by O2 concentration.
With careful consideration to adjust for optimal conditions in fabricating parts, the 0.22 MGy-EBI in O2-rich N2 atmosphere appears to be a viable method to increase carbon fiber-thermoplastic polypropylene adhesion hence friction force to prevent fiber pull-out increasing the σb over the untreated enhancing reliability and safety of the PP-CFRTP.
To increase adhesion of carbon fiber to be difficult to adhere thermoplastic polypropylene, a new process of internal activation was achieved by applying electron beam irradiation in an O2-rich N2 atmosphere to interlayered carbon fiber chopped strand matts. This was prior to assembly with PP sheets, and one directional hot-press under 4.0 MPa at 473 K for 1 min. Specimens were 3 CF-CSMs interlayered between 4 PP sheets with layup [PP]4[CF]3.
The authors wish to thank Mr. Yasuo Miyamoto (ESR), Ryo Nomura, Anna Takahashi, Naruya Tsuyuki, Daisuke Kitahara, Sagiri Takase, and Prof. Yoshihito Matsumura of Tokai University for their useful help. Our sincere gratitude also goes to Eye Electron Beam Co, Ltd. (Ghoda, Saitama, Japan) for their support with this work. This work was partly supported by the JSPS Core-to-Core Program and A. Advanced Research Networks, “International research core on smart layered materials and structures for energy saving”.