2019 Volume 60 Issue 8 Pages 1561-1570
2019 Volume 60 Issue 8 Pages 1561-1570
Mg-based hydrides have been extensively studied in the last 20 years due to its great potential as hydrogen storage materials, especially for stationary applications. Severe plastic deformation (SPD) can be used to produce Mg-based materials for hydrogen storage applications, with good activation (first hydrogenation) and H-absorption/desorption kinetics, combined with enhanced air resistance. Both advanced (e.g. high-pressure torsion, equal-channel angular pressing) and more conventional (e.g. cold rolling, cold forging) techniques were investigated as means of production of bulk samples with refined microstructures and controlled textures. Depending on the processing parameters, SPD or SPD-like techniques can produce sub-microcrystalline or even nanocrystalline structures, with a fair level of dispersion of the additives and high level of the desired [0002] fibre type texture. In this review we discuss how the processing of hydrogen storage materials by SPD techniques matches the following desirable aspects: fast kinetics (many interfaces – nano-grains and additives), easy activation (clean surfaces, interfaces, and adequate texture) and thermodynamic stability (alloying; synergy between phases in composites). The results suggest new and in most cases simpler and cheaper alternatives to produce hydrogen storage materials with proper hydrogen absorption and desorption kinetics and, in the case of composites, lower hydride stability.

Hydrogen storage in metal hydrides has been extensively researched in the last 20 years due to the high potential to be used as a hydrogen storage material. MgH2, for instance, have an outstanding hydrogen capacity of 7.6 mass% besides many other attractive characteristics in comparison with the existing alternatives for hydrogen storage.1)
However, intensive practical application of metal hydrides still faces substantial challenges as the hydrides that present high volumetric or gravimetric storage capacities typically are also thermodynamically very stable, which result in high desorption temperatures. On the other hand, hydrides that have appropriated H-absorption/desorption temperatures near room conditions (i.e., lower thermodynamic stability), present relatively low hydrogen storage capacity.
Other important limiting issues include the poor kinetics for the metal-metal hydride transformations and the difficulties for the so-called activation (first hydrogen absorption). Finally, most metal hydrides typically present low resistance for air contamination (O2, H2O, and so on).
So far, such challenges have been dealt with the following strategies; (i) reduction of the crystallite size that shortens diffusion paths and also destabilizes the metal hydride; (ii) addition of “catalytic” elements or compounds, which are considered to favour hydrogen dissociation and recombination; (iii) alloying the hydride to weaken metal–H bonds; (iv) increasing the density of “internal” interfaces and defects; (v) improving surface properties, which can be also accomplished by the use of “bulk” material instead of powders and (vi) using composite materials.
Most of these strategies were associated with processing by high-energy ball milling (HEBM), which resulted in highly reactive nanocrystalline powders. To control the surface reactivity (and easy contamination that comes with it) and to evaluate alternatives for scaling-up production, the methods of severe plastic deformation (SPD) and/or extensive plastic deformation have been used in many systems for hydrogen storage.
Because SPD processing or extensive plastic deformation routes are capable of producing ultra-fine grained (UFG) or nanocrystalline materials from conventional coarse-grained bulk metals they are of great interest for hydrogen storage since the resultant alloys also present a high density of defects, such as dislocations and vacancies, which, together with the increase in the interface area, have a positive effect on the kinetics of hydrogen diffusion.
In fact, if we consider the advantages of processing facility and low costs together with resistance to air environment in a material that still present excellent kinetics for hydrogen absorption and desorption, then SPD or extensive plastic deformation routes become very competitive in comparison with high-energy ball milling. Such advantages come in addition to the observations that commercial Mg alloys (such as ZK60) have good hydrogen storage capacities and kinetics that will depend on the processing conditions. Such alloys can be processed by SPD or extensive plastic deformation without or with further steps of pulverization, such as, by filing, turning or short-time high-energy ball milling.
In this review, we will focus on the use of such alternative processing routes to produce “bulk” (as compared with powders) Mg and Mg-based alloys and composites suitable to form hydrides. We will point out how the processing of Mg, MgH2 and Mg-based composites by SPD methods could match the following desirable aspects for a hydrogen storage material: fast kinetics (which is helped by large density of surfaces and interfaces, such as nanograins and presence of additives or second phases); easy activation (helped by clean surfaces, internal interfaces and adequate texture) and (low) thermodynamic stability (via alloying and synergy between different hydride phases in composites).
HEBM is undoubtedly the most-used technique to produce magnesium hydride and nanocomposites based on MgH2. Such nanocomposites powders may have incorporated various kinds of additives such as transition metals, transition metal oxides, fluorides and so on. The nanometric powders produced by such a technique can display the fastest hydrogen absorption kinetics at 300°C or even at lower temperatures than conventional milled powders and may take just some minutes to complete reactions of absorption and desorption.
These results are primarily associated with nano-sized grains of the MgH2 or its composites, and the presence of distinct types of lattice defects (grain boundaries, dislocations, stacking faults, and possible amorphization in some regions) that may improve the hydrogen sorption characteristics. The drawbacks associated with HEBM processing are the time and energy consuming, and the high reactivity of the powders, which must then, be handled under a protective atmosphere.
Both Mg and MgH2 powders have been processed by high-pressure torsion (HPT) aiming to reduce the crystallite size, generate defects and promote consolidation of the powders, which clearly improve surface resistance to atmospheric gases. With the same purposes, powders have also been processed by extensive cold rolling (CR) and accumulative roll bonding (ARB).
Figure 1 shows simple schemes for such processing; in the case of HPT, Fig. 1(a), the resulting sample is in the form of fully compacted disk; rolling powders in both vertical or horizontal mill result in compacted flakes.2)

(a) Typical HPT facility for processing of thin disks or powders;2) (b) scheme of a vertical rolling machine; (c) scheme of a horizontal rolling machine.
Leiva et al.3) used HPT to process a mixture of MgH2 and Fe and observed a reduction in the crystallite size of MgH2 equivalent to the ones obtained by high-energy ball milling. However, the kinetics of absorption/desorption could only be improved to the level of powder hydrides after a short time (∼30 min) HEBM processing, which broke the compact disks into small pellets. The final broken pellets contained many cracks, and the free surfaces associated with them were most likely responsible for improving the kinetics of hydrogen absorption/desorption.
Figure 2 shows in (a) the resulting MgH2+5% Fe disk after HPT processing of the MgH2 and Fe powder mixture. Figures 2(b) and (c) show scanning electron microscopy (SEM) images of the as-consolidated disk taken under different magnifications; and in (d) the desorption kinetic curves under vacuum at 350°C, for the three tested conditions. An improvement of desorption kinetics is clearly observed after HPT processing followed by a short time HEBM.

Powders of MgH2+5% Fe processed by HPT (5 GPa, five turns). (a) Photograph of a disk obtained after processing; (b) and (c) BSE/SEM images of an as-consolidated disk taken under different magnifications; (d) desorption kinetic curve under vacuum at 350°C. From Ref. 3).
Skripnyuk et al.4) used ECAP to process Mg-2 mass% multiwall carbon nanotubes composite and reported faster hydrogen absorption kinetics at 300°C for the composite than for pure Mg processed by ball milling or ECAP. Such fast kinetics were associated with fast diffusion of hydrogen through the carbon nanotubes, which after ECAP, were reduced in size and contained different types of defects.
Lang and Huot5) showed that cold rolling of commercial MgH2 enhanced hydrogen sorption properties by improving the sorption kinetics. Such effect was associated with the nanocrystalline structure, which was formed after only a few rolling passes, and the increased density of defects. The results indicated that cold rolling could be equivalent to ball milling in terms of microstructural refinement, a result, which already had been observed by other research groups.3)
Extensive CR and cold forging (CF) of MgH2–Fe mixtures6,7) were also very effective in decreasing the crystallite sizes to the nanometric range and in assuring a relatively homogeneous distribution of Fe particles resulting in compacted flakes. Activation was slow in the as deformed flakes but improved significantly with additional short time milling, most likely due to the creation of cracks or new surfaces, in the flakes.
Figure 3 shows transmission electron microscopy (TEM) micrographs of the nanostructured MgH2 prepared by (a) cold rolling and (b) cold forging. Figure 3(c) shows the DSC curve for the as-received MgH2 and after CR and in (d) the H-sorption kinetics at 350°C of MgH2 in three different conditions: after CR, after CF and MgH2 +5% Fe after CF. It is important to notice how the presence of Fe improved the desorption kinetics, an effect most likely associated with the increased interface area.

Powders of MgH2 processed using 5 passes of CR and powders of MgH2 and MgH2 +5% Fe using 5 passes of CF (a) Dark field TEM image of MgH2 after CR. (b) Dark field TEM image of MgH2 after CF. (c) DSC curve for the as-received MgH2 and after CR. (d) H-sorption kinetics at 350°C of MgH2 after CR, after CF and MgH2 +5% Fe after CF (absorption at 20 bar and desorption at 0.6 bar). From Ref. 6).
The importance of small grain sizes to enhance hydrogen storage properties in consolidated bulk samples was clearly shown by Asselli et al.8) after processing MgH2 by extensive forging and increasing processing energy, either by many forging passes or by increasing the energy transferred during forging, resulting in faster kinetics of hydrogen absorption and desorption. However, since forging was carried out in air, the initial enhancement of kinetics associated with higher processing energy was accompanied by higher reactivity of samples and higher level of oxidation, with the formation of MgO and consequent degradation of the hydrogen capacity and kinetics. Therefore thicker samples with lower oxide content presented better hydrogen storage properties.
Extensive cold rolling was also used to process MgH2 powders containing different types of additives, such as Fe; FeF3; Fe2O3; Nb and Nb2O5.9) The authors confirmed the effectiveness of cold rolling in reducing crystallite sizes and to uniformly distribute the additive particles. Interestingly, only a modest effect due to the presence of the additives was observed during hydrogen absorption, contrary to what was observed during desorption, when the additives played an important role, with the best effects being observed for the samples containing Nb2O5 and FeF3.
Another interesting route to processing MgH2 powders, this time containing FeF3 as an additive, was performing cold rolling just after short-time processing by HEBM.10) The initial short time HEBM step before CR resulted in finer additive particle size distribution and better level of mixing between MgH2 and FeF3 as compared to direct cold rolling, and strong texture in the β-MgH2 phase. The excellent values of desorption kinetics that were measured were associated with a much finer additive particle size, better distribution and better level of mixing.
Panda et al.11) observed an interesting effect of the type of the original powder processed by HPT on the hydrogen storage properties of Mg. Consolidation of condensed ultrafine Mg powder particles resulted in faster absorption kinetics as compared with the HPT sample obtained from atomized micro-sized powder. However, the sample obtained from the atomized powder was able to absorb more hydrogen and showed faster desorption kinetics indicating different responses in terms of grain refinement after HPT and dispersion of MgO.
In another investigation, Panda et al.12) reported a more detailed study concerning the microstructural development during HPT using two different initial powders (atomized and condensed) and observed substantial differences in the development of the microstructure, texture, local strain heterogeneities, and hardness in the two types of products. Such differences resulted in a significant effect on the hydrogen activation kinetics to form hydrides.
The use of protective atmosphere to process MgH2 by cold rolling was reported by Márquez et al.;13) the results indicated the possibility to increase the number of rolling passes and, therefore, decrease the crystallite sizes, without any noticeable surface contamination. This processing route was considered an excellent alternative to ball milling since the resulting compacted flakes presented faster and equivalent good kinetics of absorption/desorption and high capacity as compared with milled powders, in addition to better resistance to surface contamination.
HPT processing of MgH2 was also used to transform the tetragonal structure to the gamma orthorhombic structure, which resulted in decreasing the dehydrogenation temperature by 80 K.14)
Mg2Ni powders were also compacted by HPT.15) The hydrogen absorption and desorption behaviors were characterized by high-pressure calorimetry. From the values of hydrogenation/dehydrogenation reactions enthalpies, the authors suggest that HPT promotes the destabilization of the metal-hydrogen bonds.
In the case of the complex Mg2FeH6 hydride, HPT processing was used to consolidate the powder and resulted in attractive properties concerning absorption and desorption kinetics, which were close to the ones observed in Mg2FeH6 powders.16) The apparent advantage of such consolidation by HPT was to obtain a hydride with reduced reactivity with the contaminants present in the air.
Thus, consolidation of magnesium hydride powders by different SPD methods are generally advantageous for improving kinetics as well as for enhancing hydrogenation activity, air-resistance, and, as significant as these effects, even for synthesizing new hydrides with nano- or ultrafine grains with a high density of lattice defects.
Although HPT has also been used to process and transform bulk Mg-based material for hydrogen storage (as will be indicated below) processing such type of material is more conveniently performed by other SPD techniques or extensive plastic deformation routes.
SPD and extensive plastic deformation methods give the ability to transform conventional coarse-grained metallic materials into ultrafine-grained or nanocrystalline ones under high hydrostatic pressure and at moderately low deformation temperatures.17–19) Such methods can produce many defects in the crystalline lattice such as vacancies and dislocations, which has a positive effect on the hydrogen diffusion kinetics. Since the final product is still a bulk sample (even though, sometimes in the shape of flakes), one of the primary interest in such processing is to develop more air-resistant materials. Despite the lower specific surface area in comparison with powders, materials processed by SPD still keep attractive H-sorption kinetics.
A scheme of ECAP processing is shown in Fig. 4.20) The ECAP die is composed of two channels of the same cross-section intersecting to form a corner, as presented in Fig. 4(a). Φ and Ψ represent respectively the channel angle and the curvature angle, which can be varied accordingly to the desired imposed deformation. Specimens are subjected to a pure shear strain when pushed through the die by a plunger, inflicting straining without any change in their cross-sections. In this way, having the same cross-section, samples can be deformed to very high strains leading to intensive grain refinement, in some cases to the nanoscale.19)

Processing of Mg-based alloys or composites for hydrogen storage by SPD was pioneered introduced by Skripnyuk et al.21) processing, in this case, a ZK60 alloy. Other SPD works followed in Mg–Mm–Ni22) and Mg–Ni alloys.23) The main attractiveness for the use of SPD was the possibility to control the microstructural evolution, such as decreasing grain sizes, increasing the density of defects, inducing the formation of textures, designing of special types of grain boundaries and increase the degree of supersaturation. In the case of the Mg–Ni alloy, the improvement in hydrogenation performance was associated with the chemical inhomogeneity induced by ECAP processing.
Other processing routes involving extensive plastic deformation in Mg-based alloys for hydrogen storage soon followed; such as extensive rolling or accumulative roll-bonding (ARB) to prepare laminate composites.24–27)
In the case of ARB, the specimens (different compositions or not) are stacked and conventional rolling are repeated several times, Fig. 4(b). The two plates are joined together by stacking and rolling. After each rolling pass, the folded material is split into two halves, and the mechanical processing is repeated several times to finally obtaining an ultra-fine microstructure.
Following the first attempts to process bulk Mg and Mg-based alloys, many other successful reports described the improvement in the kinetics of hydrogen absorption and desorption in Mg-based system by using different SPD or extensive plastic deformation techniques.28–42)
Wang et al.43) provided a detailed review concerning the factors that affect the hydrogen storage properties of Mg alloys processed by ECAP, emphasizing, like many other authors, the effect of grain sizes, lattice defects, catalysts, and textures introduced by ECAP process.
Skripnyuk et al.44) evaluated the refinement of ZK60 alloy by ECAP, HEBM and by a combination of both methods. The highest rate of hydrogen desorption was observed for the alloy processed by a combination of ECAP and HEBM; although the alloy processed by ECAP alone showed only slightly slower desorption kinetics.
ECAP was also applied to as-cast eutectic Mg–Ni alloy,45) resulting in important refinement of the Mg and Mg2Ni grains in addition to supersaturation of Ni in the Mg grains. The deformed samples presented a gravimetric hydrogen storage capacity of about 6 mass%. ECAP also affected positively the equilibrium hydrogen desorption pressure, which increased with the increasing number of passes.
The same group29) used ECAP to process ZK60 alloy. They observed an increase in hydrogen desorption pressure and acceleration in the hydrogen desorption kinetics, which was associated with the microstructure refinement and an increase in the density of defects due to the ECAP processing.
Magnesium ZK60 alloy was also processed by ECAP by Krystian et al.46) who observed that the resulting bulk nanostructured alloy meet or even exceed the absorption/desorption kinetics of their ball-milled counterpart. They also discussed the effect of grain size, surface oxidation and addition of metallic chromium as a catalyst on the storage capacity and kinetics in the bulk alloy. An impressive result of such work was the long-term cyclic sorption/desorption tests up to 1000 cycles when no deterioration in storage capacity or kinetics was observed.
A clear indication of the important effect of texture and the presence of free surfaces was observed in the AZ31 alloy processed by ECAP and cold rolling.47) Figure 5 shows in (a) the ECAPed processed samples and in (b) and (c) the resulting microstructures after processing at 200°C and 300°C, respectively. In Fig. 5(d), the XRD patterns show the effect of cold rolling in establishing the (002) texture.

AZ31 alloy processed by ECAP and ECAP plus cold rolling. (a) Photograph of the specimens after two passes of ECAP at the indicated temperatures; (b) and (c) TEM bright field images after ECAP processing: (b) ECAP at 200°C; (c) ECAP at 300°. (d) XRD patterns comparing the conditions of as-received (extruded), after ECAP at 200°C, and after ECAP at 200°C followed by cold rolling. From Ref. 47).
Using severe and extensive plastic deformation routes to process commercial Mg, Leiva et al.47–49) observed that the presence of nanograins is not enough to result in an adequate activation of bulk samples; the presence of free surfaces (from cracks, for example) and texture (from additional cold rolling) were found to be relevant for activation kinetics. They observed that one good condition for hydrogen storage properties was associated with combined processing of melt-spinning (MS) followed by cold rolling that is, cold rolling of an already refined microstructure. These results pointed out important features to be controlled in bulk samples such as the deformation texture and the surface to volume ratio. The same tendency was observed in melt-spun Mg–Ni alloy;50) cold rolling of the MS ribbons improved the kinetics of hydrogen absorption and desorption significantly.
Huot et al.51) reviewed the use of ECAP and CR for enhancement of hydrogen sorption properties of magnesium and magnesium alloys. The effects of the processing route and type of texture they produced were associated with the hydrogen storage properties.
Botta et al.52) also reported the positive effects of texture, small grain size and free surfaces (or interfaces) for the hydrogen storage properties, after processing commercial Mg by melt-spinning, HPT and CR.
Processing of commercial Mg by cold rolling under protective atmosphere also resulted in attractive microstructure for hydrogen storage properties.53) Figure 6 shows the hydrogen activation kinetics curves for Mg samples processed by CR and CR plus short-time processing by HEBM. Excellent absorption capacity was observed for the sample processed by extensive CR under an inert atmosphere, which resulted in a refined microstructure, a large density of defects, strong texture, and no oxides or hydroxide layers formation, followed by additional short-time milling, which increased the surface area.

Hydrogen activation kinetic curves at 623 K under 2 MPa of H2 pressure for commercial Mg processed by CR and CR + HEBM.53)
The advantage of using CR to induce appropriate texture in commercial Mg was also observed by Lima et al.54) In this case, ECAP combined with CR samples presented faster hydrogen absorption kinetics than samples processed only by ECAP. This result was associated with the favorable (002) texture and a higher density of defects after the combined processing.
In a detailed analysis of the effect of texture for the hydrogen storage capacity in Mg and Mg alloys Jorge Jr. et al.35,36,55) were able to show a direct correlation of the (002) texture with higher capacities, faster kinetics and lower hydrogen desorption temperatures in commercial Mg,35) in AZ31 alloy36) and in AM60D and AZ91 alloys.55)
Combined processing by ECAP and CR was also used in a modified ZK60 alloy containing 2.5 mass% Mm.56) The authors observed that the combined ECAP + CR processing was responsible for relatively easy activation to reach 80% of theoretical capacity. The resulting microstructure was refined and with a wide distribution of sizes of the Mm-containing intermetallic second phases, which was found essential for the activation.
The same ZK60-2.5% Mm alloy were produced by two different routes involving melt-spinning, ECAP and CR.57) Similar storage properties were obtained with ECAP+CR and MS+CR after activation, and the authors suggested the ECAP route to be more attractive due to its simplicity and to the higher air resistance that is attained in the samples.
Cold rolling also had a positive impact on 2Mg–Fe mixtures consolidated by hot-extrusion.58) Figure 7 shows a TEM image of a hot-extruded and cold rolled compacted sample, after two absorption/desorption cycles. Optimized results were observed for the combined processing, which was explained by the refined grain sizes, favorable (002) texture of Mg and the presence of residual Fe.
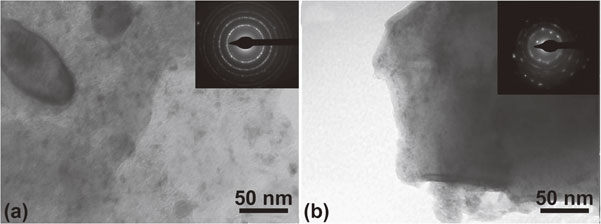
Bright field TEM images of hydrogenated 2Mg–Fe: (a) extruded and (b) extruded/rolled. Insets represent selected area diffraction patterns indicating the refined microstructure and also some texture in (b).
The same conclusions pointing out the importance of texture and fine distribution of additive elements or phases were also obtained in mixtures of Mg–Fe containing carbon nanotubes processed by hot extrusion and cold rolling.59)
The combined processing of CR and ARB was used in Mg–Fe mixtures60) resulting in compacted flakes containing (002) texture, similar to CR processing. However, better hydrogen sorption properties were observed after the combined CR+ARB processing, which was associated with grain size reduction, increased density of cracks and better distribution of Fe particles.
Popilevsky et al.61) used ECAP to process Mg–10 mass% Ni alloy and reported an initial faster hydrogen absorption for the ECAPed processed alloy in comparison with the as-cast alloy, which however was lost in subsequent cycles. Such behavior was associated with the microstructural modification during the hydrogenation cycles including the formation of large faceted Mg crystals, cracking, accumulation of plastic strain in the Mg crystals, and redistribution of the dispersed particles of Mg2Ni phase in the partly hydrogenated alloys.
A processing route combining ECAP and ARB was used in a commercial ZK60 magnesium alloy.40) Hydrogen capacity was very low in the ECAPed sample and was significantly improved by the ARB step, although the expected capacity was not reached. Crushing of the samples further improved the hydrogen absorption capacity; suggesting the importance of refined microstructure and adequate sample morphology with enough free surfaces for hydrogen interaction.
Grill et al.62) reported the long-term hydrogen storage in Mg and ZK60 after severe and extensive plastic deformation, comparing samples processed by HPT and CR. HPT processed samples were observed to maintain much better stability than CR samples both for kinetics and hydrogen storage capacity after many absorption/desorption cycles. In the CR samples, MgH2 nucleation was followed by growth of MgH2 domains, which was not observed after HPT processing. The authors discussed the results considering the different density and stability of defects in the different processing routes, and how they can act as nucleation sites.
The effect of lowering the sample temperature during rolling passes was tested in AZ91 alloys.63) Low temperature rolling (LTR) resulted in better H2 absorption/desorption kinetics and capacity as compared to samples processed by conventional CR. The excellent properties exemplified in Fig. 8 were associated with microcracks and a large amount of exposed interfaces and formation of [0002] texture after CR.

Activation kinetics curves for Mg-AZ91 samples after CR and LTR processing conditions. From Ref. 63).
As already mentioned in the case of MgH2 powders, HPT processing was also used to produce consolidated bulk samples of Mg–Fe mixtures.64) Hydrogen absorption/desorption kinetics and hydrogen desorption temperatures were improved by the presence of Fe particles suggesting the relevance of internal interfaces in the bulk samples.
HPT processing was used to design stacking faults containing microstructure in Mg2Ni intermetallics.65) Comparison of hydrogen storage properties in coarse-grained, nanograined and stacking faults containing alloys indicated the important effects for the hydrogenation kinetics due to the presence of grain boundaries and stacking faults, which can then also act as quick pathways for the transportation of hydrogen in the hydrogen storage materials.
HPT processing was useful to synthesize new Mg-based alloys designed using first-principles calculations to present low hydrogen binding energy and room temperature hydrogen storage properties.66) HPT also proved to be a useful route to form interesting metastable phases in the Mg–Ti system67) and new nanostructured phases in the magnesium–zirconium system.68)
Severe or extensive plastic deformation has been used more recently to process Mg-based composites. MgH2–LaNi5 mixture was processed by cold rolling under controlled atmosphere resulting in compacted composite flakes,69) which presented faster kinetics and reduced desorption temperatures in comparison with MgH2.
Figure 9 shows the absorption kinetics curves for the MgH2+1.5 mol% LaNi5 composite during 10 cycles of hydrogen desorption/absorption. Absorption was performed at only 100°C, which is considered as a low and attractive temperature for absorption. These results show excellent stability of the samples for cycling, which was undoubtedly helped by the compaction of the powders during cold rolling and the clean surfaces due to the controlled atmosphere during processing.

MgH2–LaNi5 composite processed by cold rolling at a controlled atmosphere showing results of 10 cycles of absorption.69)
One of the first uses of filing to prepare samples for measuring hydrogen storage properties was reported by Krystian et al.30) in ZK60 alloys processed by ECAP. Although the authors did not discuss if such processing had some effect in the microstructure after ECAP, filing positively affected absorption and desorption kinetics due to the increased surface area to volume ratio.
More recently Asselli et al.70) evaluated the effect of the microstructure and morphology of Mg samples after processing by ARB, filing, and a combination of both processes. Faster activation and enhanced hydrogen storage properties were observed with the combined ARB, which refined the microstructure of filings, thus increasing the surface/volume ratio of the samples.
The same group evaluated the effect of the filing tool on the morphology and hydrogen storage properties of Mg chips,71) and observed that chips with a larger surface area resulted in faster hydrogenation and dehydrogenation kinetics, which is an expected result, although, probably, in this case, an essential effect of the microstructure refinement should also be considered.
A different approach to scale-up the production of Mg alloys for hydrogen storage was suggested by Silva et al.72) An as-cast ZK60 Alloy was processed by friction stir welding (FSW) resulting in an ultrafine grained and fully recrystallized microstructure, which also had a fine distribution of the intermetallic phases. Chips were then obtained from the as-cast (AC) and from the stir zone (SZ) regions to evaluate the hydrogen storage properties. Figure 10 shows SEM images of the filings removed from the AC regions in (a) and (c) and SZ region in (b) and (d). Hydrogenation kinetics and capacity were very attractive in chips filed from the SZ region, contrary to what was observed in chips removed from the AC region. The improved results in chips from SZ regions were associated with the microstructural refinement, intermetallic fragmentation, which increased the interface area and higher surface area fraction.

SE-SEM images of the filings: (a) from the AC sample, and (b) from the SZ sample. (AC - as-cast from the base metal and SZ - stir zone). Kinetic curves for the filings obtained from AC and SZ samples: (c) second absorption, and (d) third desorption.72)
Different processing routes involving SPD or extensive plastic deformation are being used to produce nanostructured Mg-based materials for hydrogen storage. Compared to HEBM, the SPD techniques are faster and more straightforward and therefore are possibly less costly. The processing conditions can be adjusted to allow full H-absorption/desorption to occur in a few minutes; however, in general, operational temperatures around 350°C are needed for this, in contrast to the typical value of 300°C for ball-milled powders.
The Mg bulk material obtained by SPD-type processing can present many characteristics considered relevant for hydrogen storage. A very refined microstructure is produced, in some cases reaching the nanoscale, in terms of grain size and dispersion level of a second phase (catalyst). Therefore, an important fraction of grain boundaries and interphase contours are present in the material. These interfaces act as faster diffusion paths for hydrogen and thus enhances the kinetics of H-absorption/desorption. SPD can produce cracked Mg-materials, with a larger exposed surface and therefore more reactive regarding H2. The crystallographic texture can be, at least up to some level, controlled, using different processing routes or conditions. This feature has shown to be essential for shorter activation time.
ECAP processing was explored in several studies about Mg alloys for hydrogen storage, due to the potential of this technique for mass production of bulk metallic materials with fine microstructures. The processing temperature has shown to be a key issue in these studies, since in order to allow significant straining, moderate temperatures were needed to provide plasticity; on the other hand, these moderate temperatures lead to recovery and recrystallization processes, and therefore limited grain refinement.
The use of the more simple techniques of CR or ARB provides more attractive hydrogen storage properties for Mg and its alloys, especially enhanced activation kinetics. The extensive deformation by rolling at room temperature produces samples in the form of thin foils, with a large amount of defects, very small grain sizes, and intense fibre texture in the [0002] direction. These characteristics favour the reactivity with hydrogen. The morphology of the rolled samples, which consists of cracked foils or flakes with thickness typically around 0.1 mm, is particularly interesting, resulting in a combination of attractive H-absorption/desorption kinetics with elevated air-resistance. ECAP and CR were coupled to maximize microstructural refinement. Also, a short final HEBM step has shown to be very beneficial for the hydrogen storage properties.
Magnesium hydride with or without additives can be used as starting material for SPD processing instead of metallic Mg or its alloys. A much more intense grain refinement is obtained when MgH2 is used, reaching typically a few tens of nanometers, which is compatible with the level of refinement obtained by HEBM.
Many details remain unexplained regarding the correlations between structure and properties of SPD Mg-based materials for H2 storage. These include understanding the different roles played by the free surfaces (such as external surfaces and crack surfaces) and the internal interfaces (such as grain or interphase boundaries) during activation and for the kinetics of hydrogen absorption and desorption. Furthermore, several challenges are still faced to improve the attractiveness of the SPD-processed alloys or composites for hydrogen applications. Some of these challenges include the critical evaluation of the different routes (or combination of routes) that result in adequate defect structure, how each structure evolves during hydrogen absorption/desorption cycling and which route presents the best compromise of costs, up-scaling and microstructure control.
On the other hand, new and interesting results are continuously being obtained by the use of unconventional processing routes, as FSP, for example; by the adaptation of more conventional processes as CF or filing; or by the variation of significant processing conditions, e.g. performing cold rolling under protective atmosphere.
One can conclude that the development of SPD Mg-based materials for H2 storage is still a very relevant topic of applied research. The examination of the more recent articles in the field reveals that new studies about the pulverization of these highly deformed alloys, as well as the elaboration of Mg or MgH2-based composites with relevant amounts of low temperature hydride formers such as TiFe or LaNi5, should bring consistent advances in the level of the hydrogen storage properties.