2019 Volume 60 Issue 9 Pages 1828-1832
2019 Volume 60 Issue 9 Pages 1828-1832
Mechanical properties relating to the thermally activated process of yielding was investigated in Ti–6Al–4V with a bimodal microstructure, consisting of both primary alpha grains and lamellar colonies of secondary alpha/beta lamellae. The temperature dependence of yield stress, effective stress, activation volume, and activation enthalpy were investigated between 77 K and 650 K. The yield stress and effective stress decreased with increasing temperature. It was found that the temperature dependence of activation enthalpy for yielding shows values between those obtained from basal slips and prismatic slips investigated in single crystalline α-titanium. This suggests that the thermally activated processes which control the yielding of bimodal Ti–6Al–4V is the combination of basal and prismatic slips.

Ti–6Al–4V is one of the most prominent, efficient, and commercially used titanium alloys especially employed in aerospace industries. Titanium alloys are superior to other materials in terms of high specific strength (high strength-to-weight ratio), excellent mechanical properties, and corrosion properties majorly at both ends of operating temperatures such as low temperatures (cryogenic tanks, superconducting magnet support systems), body temperature (biomedical materials), and high temperatures (turbines, jet engines).1–3) Supreme mechanical properties can be obtained in Ti–6Al–4V by controlling the microstructure.4–6) One of the major attractions for Ti–6Al–4V is that it is possible to obtain various types of microstructures. For example, Ti–6Al–4V is processed into fully alpha primary nodular grains, widmansttatten structure, bimodal holding both primary alpha, and lamellar colonies consisting of secondary alpha and beta phases.7–13)
Number of studies have been attained on mechanical properties of pure titanium.14–19) Macroscopic deformation of the materials is associated with the yielding of individual grains, which depends on grain orientations, grain sizes, and so on. The major slip systems of hexagonal close-packed (hcp) crystals are basal, prismatic, and pyramidal20–22) as shown in Fig. 1. In pure titanium, the prismatic slip is the easiest to be activated amongst all slip systems at room temperature and is prominent in slip deformation. Two possible Burgers vectors are $\langle \text{a}\rangle (a/3\langle 11\bar{2}0\rangle )$ and $\langle \text{a} + \text{c}\rangle (a/3\langle 11\bar{2}0\rangle + c\langle 0001\rangle )$, where a and c are the lattice constants along the a-axis and c-axis, respectively. The Burgers vectors of $\langle \text{a}\rangle $, and $\langle \text{a} + \text{c}\rangle $ are also shown in Fig. 1. Dislocations with the Burgers vector of $\langle \text{a}\rangle $ have the shortest length among the possible Burgers vectors, so that it is considered to be the essential one in the prismatic slip.23–30)

Possible slip systems in Titanium.
Bimodal structure has been more complex among the wide ranges of microstructures, for example, the combination of pure α, α + β, and widmanstatten are available in Ti–6Al–4V. The complexity arises essentially from the combination of phases comprises of different crystal structures such as α (hcp), and β (body-centred cubic: bcc). Mechanical properties in Ti–6Al–4V with bimodal structure of primary α nodular grains and α/β lamellar colonies are influenced by the microstructures as the mechanical properties of primary α nodular grains and α/β lamellar colonies are not the same.31)
In Ti–6Al–4V with bimodal microstructure, the control mechanism behind the temperature dependence of the yield stress is more complicated than α-Ti because the temperature dependence is influenced by the thermally activated process of dislocation glide in both α and β phases where the effects of solute atoms such as Al, V, and O are essential. It was reported that both solute oxygen and aluminium atoms contribute in the changes of slip systems in α phase as they affect the CRSS by modifying the c/a ratio. The CRSS of prismatic slip increases as the concentration of aluminium increases. The increase in the CRSS of prismatic slip is much higher than that of basal slip,32–34) which leads to the activation of basal slips. On the other hand, solute vanadium in β phase is expected to decrease the yield stress of the phase by the decrease in the elastic modulus with vanadium.3)
In addition to the effect of microstructure and solute atoms, the effect of temperature on mechanical properties is also very important. Various researches were performed to improve the mechanical properties of Ti–6Al–4V in different temperature ranges and strain rates varying aging and annealing temperatures of samples. Gysler et al.35) studied the temperature dependence of yield stress in aged Ti–6Al–4V at between room temperature and 773 K. They showed that interstitial atoms influenced the temperature dependence of yield stress up to 573 K while there is no temperature dependence observed above 573 K. Lee et al.36) investigated deformation mechanisms at high strain rates, and temperatures between room temperature and 1373 K. They mentioned that both the strain rate and temperature could affect both flow stress and work hardening coefficient of Ti–6Al–4V at high strain rates. Flow stress is much more sensitive towards strain rate than temperature and increases linearly with strain rate, which suggests the deformation process is controlled by thermally activated processes. De Meester et al.37) studied the deformation kinetics of Ti–6Al–4V at low temperatures (42 K to 760 K) with additional interstitials. They showed that the rate-controlling mechanism in Ti–6Al–4V is the thermally activated process of dislocations overcoming interstitial solutes. The strengthening mechanism of the alloy is governed by substitutional solid solution atoms such as Al and V. Follansbee et al.38) used Kocks-Mecking model to explain the changes in deformation mechanisms at low temperatures between low strain rates and high strain rates. The model gives phenomenological view of thermally activated interactions of dislocations. They found that the rate of strain-hardening at low temperatures are quite higher than those expected. It is due to the contribution from athermal component. Although substantial studies were continuing on studying mechanical properties of Ti–6Al–4V, less focus has been given to the dislocation activity by precise altering of temperature range in bimodal microstructure which was emphasized in present study.
To study thermally activated processes, the yield stress is divided into two components. One is effective stress which is temperature dependent, another is athermal stress which is temperature independent. The effective stress is obtained by subtracting the athermal stress from the yield stress at a given temperature as below.39)
| \begin{equation} \sigma_{e} = \sigma_{y} - \sigma_{\textit{ath}}, \end{equation} | (1) |
In the yield process which is controlled by dislocation motion, the strain rate at yielding is given by the Orowan equation:
| \begin{equation} \dot{\varepsilon} = \rho bv, \end{equation} | (2) |
| \begin{equation} v = v_{0}\left(\frac{\tau_{e}}{\tau_{0}} \right)^{m}\mathit{exp}\left[- \frac{\Delta H(\tau_{e},T)}{kT}\right], \end{equation} | (3) |
In the present study, therefore, the temperature dependence of mechanical properties of Ti–6Al–4V with the bimodal structure were investigated. The temperature dependence of yield stress, effective stress, activation volume, and activation enthalpy were measured from the results obtained by tensile tests. The temperature dependence of activation enthalpy for yielding was investigated in detail in order to discuss the thermally activated process which controls the yielding in the bimodal Ti–6Al–4V employed in this study.
Titanium alloy with 6 mass% of aluminium and 4 mass% of vanadium was employed in this study. A hot forged billet was heat treated at 1213 K for 2 hours followed by normalising at 978 K for 3 hours, and then air cooled. An electric discharge machine (Mitsubishi Electronic, MV1200R) was used to make tensile samples and metallography ones. Tensile tests were conducted in the temperature range between 77 K and 650 K to determine the temperature dependence of yield stress, effective stress and activation volume. The thickness of tensile specimens was 1 mm. The length and the width of the parallel portion of the tensile specimen were 8 mm and 2 mm, respectively. The initial strain rate was 5 × 10−4 s−1. Microstructure was observed by using scanning electron microscopy (Hitachi, S4300) and electron backscattered diffraction (EBSD). Metallography samples were electro-polished by using electrolyte CH3OH and HClO4 of 9:1 ratio.
Figure 2(a) shows a scanning electron micrograph, indicating that the microstructure of the employed Ti–6Al–4V is bimodal, containing primary α nodular grains and α/β lamellar colonies of secondary α and β phases. The distribution of nodular grains and lamellar colonies have nearly the same volume fractions with the average grain size of 8 µm in an equivalent circular diameter. Evolution of secondary α from primary α grains is observed as shown as circular shape in Fig. 2(b).

SEM images of the specimen employed in this study. (a) Bimodal distribution, (b) Evolution of Secondary α.
Figure 3 shows nominal stress–strain curves, indicating that yield stress decreases with increasing temperature while the total elongation has no strong temperature dependence especially at high temperatures. The reason why the total elongation shows nearly independent from temperature will not be discussed further in this paper. However, it would depend on the temperature dependence of work-hardening. The magnitude of work-hardening rate of hcp metals in stage II is one order lower than that of fcc crystals.40) It is because the number of slip systems are so limited that the dislocation density does not increase as does in fcc crystals.

Nominal stress-strain curves at different temperatures in Ti–6Al–4V.
Figure 4 represents the relationship between yield stress and temperature. Here, the 0.2% proof stress was taken as the yield stress in this study. Yield stress holds an inverse relationship with temperature although there is a strong scattering between 225 K and 300 K. Yield stress decreases with temperature, and loses the temperature dependence over at 450 K. The plateau stress obtained as the average of yield stress at above 450 K is approximately 580 MPa. The value of yield stress is divided into two components, i.e., effective stress (σe) which is temperature dependent, and athermal stress (σath) which is temperature independent. Effective stress at a certain temperature is defined as shown in eq. (1). The value of athermal stress in this study is defined as the plateau stress of 580 MPa as obtained from Fig. 4.

Temperature dependence of Yield stress in Ti–6Al–4V.
Figure 5 shows the temperature dependence of effective stress. Two 2nd-order polynomial fits in black and red curves are constructed in Fig. 5 by considering the temperature dependence altered at 325 K. It is clearly indicated that different trend of the effective stress is manifested at below and above 325 K. In addition to that, it looks large scattering at the temperature range between 225 K and 300 K. The change in the trend, and the scattering of data between 225 K and 300 K suggest the change in the thermally activated process for yielding at this temperature range. The similar characteristic of abnormality temperature range in Ti alloys is reported and summarized by Majorell et al.41) In order to assess the change in the thermally activated process, the temperature dependence of activation volume was investigated next because the activation volume is one of the most prominent parameters relating to the thermally activated process.

Temperature dependence of effective stress.
The activation volume is obtained by the multiplication of activation area and the absolute value of Burgers vector. The activation area is defined as the area where a dislocation swipes when it overcomes short range obstacles. Therefore, it gives the insight into the estimation of the thermally activated process. The value of activation volume is experimentally obtainable by the following equation with strain rate jump tests:42)
| \begin{equation} V^{*} = MkT\frac{\ln(\dot{\varepsilon}_{2}/\dot{\varepsilon}_{1})}{\sigma_{2} - \sigma_{1}}, \end{equation} | (4) |
Figure 6 shows temperature dependence of activation volume, where the y-axis was normalised by the Taylor factor (M). Activation volume increases with increasing temperature. There is a different trend is observed at the temperature range between 225 K and 300 K, i.e., an inverse temperature dependence in the activation volume. This suggests the change in the slip mechanism, which was assumed by the change in the trend of temperature dependence of effective stress, and scattering of data at the temperature range shown in Fig. 5. It also suggests that the thermally activated process which controls the yielding should change at between 225 K and 300 K. Conrad et al.43) summarised that the activation enthalpy for basal slip is higher than that for prismatic slip at any temperature in single crystalline α-titanium. Therefore, obtaining the temperature dependence of the activation enthalpy gives an insight into the controlling mechanism behind yielding in Ti–6Al–4V. Here, activation enthalpy is obtained by the following equation:34)
| \begin{equation} \Delta H = - \frac{TV^{*}}{M}\left(\frac{\partial \sigma_{e}}{\partial T} \right), \end{equation} | (5) |

Temperature-Activation volume relation in Ti–6Al–4V.

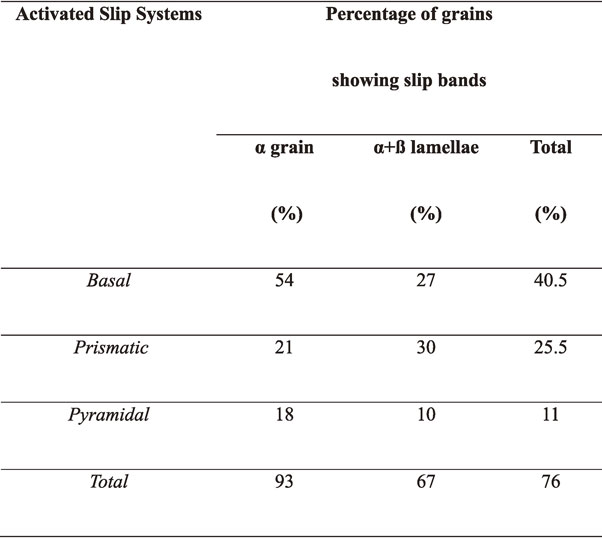
Table 1 shows the percentage of activated slip planes in α nodular and α/β lamellar grains at the total tensile strain of 0.03 deformed at 77 K investigated by using EBSD. The observed area was 8000 µm2. 93% and 67% of α grains and α/β lamellar grains show slip bands (76% of total grains show slip bands), respectively. It is to be noted that both basal slips and prismatic slips are activated in α nodular grains and lamellar colonies. It is in good agreement with the results that the activation enthalpy shows values between the results from basal prismatic slips as shown in Fig. 7, suggesting that the activation of both basal and prismatic slips in Ti–6Al–4V employed in this study. Ambard et al. studied the role of interfaces and proceeding of slips from one phase to other phase with different crystal structure at 20 K in Ti–6Al–4V. In lamellar colony, slip originates at the α/β interface and propagates into the grain by following specific burgers orientation relationships of α-β phases i.e. basal slip of α phase is parallel to the β phase slip of $\langle 111 \rangle $ {110}.47)
In polycrystalline pure titanium, the value of CRSS for basal slips is much higher than that of prismatic slips at low temperatures, which restrict the activation of basal slips in pure titanium at low temperatures. However, it was reported that the increase in the aluminium concentration in α-titanium increases the CRSS of both prismatic and basal slips.36–38) The increase rate in CRSS with aluminium for prismatic slips is much larger than that for basal slips. Then, the value of CRSS for prismatic slips becomes close to that of basal slips in Ti–6Al, which promotes the activation of basal slips even at low temperatures. Therefore, it is expected that the change in the CRSS is also seen in Ti–6Al–4V i.e. the CRSS for basal and prismatic slips in Ti–6Al–4V becomes close enough to induce the activation of basal slips even at low temperatures.
Temperature dependence of mechanical properties were investigated in Ti–6Al–4V by performing tensile tests. Following results were obtained.
This work is partly supported by the Structural Materials for Innovation of the Cross Ministerial Strategic Innovation Promotion Program (SIP) of Japan Science and Technology (JST), and JSPS KAKENHI Grant Number JP18H03848.