2020 Volume 61 Issue 10 Pages 1912-1921
2020 Volume 61 Issue 10 Pages 1912-1921
Herein, the isothermal aging behavior of copper–titanium–magnesium (Cu–Ti–Mg) supersaturated solid-solution alloys, with different compositions, under test conditions of 450°C for 100 h, has been thoroughly investigated in a comparative study using various electron microscopy and microanalytical techniques. The Vickers hardness and electrical conductivity of the ternary alloys were recorded at slightly elevated (during aging) and reduced levels than their binary counterparts without Mg doping. Hence, it is proposed that the hardness and conductivity values are approximated from the superposition effect of precipitation hardening stimulated by Ti solutes and solution hardening by both Ti and Mg solutes. Furthermore, the tensile tests for these ternary specimens have demonstrated that Mg doping has a substantial effect on the improvement of the tensile strength and fracture elongation properties of binary Cu–Ti alloys. Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy imaging combined with atomic-resolution energy-dispersive X-ray spectroscopy mapping analysis confirmed that the same metastable precipitate phase is responsible for peak hardening in ternary and Cu–Ti binary alloys. In addition, a large part of the Mg solutes is homogeneously distributed over the matrix regions, while there is also a smaller part of those present in the precipitates. The potential effects of Mg doping on the microstructures of Cu–Ti alloys were elucidated and the structural environment, which may yield relatively high mechanical properties, was discussed using the aforementioned observations.

Fig. 10 (a) Atomic-scale HAADF-STEM image of a single precipitate particle of the β′ crystal present in the Cu–2Ti–2Mg PA specimen, which was taken in the [001]α incidence of the α-Cu FCC matrix, together with (b) the digital Fourier diffractogram of the lattice image in the inset.
Modern automotive and computer technologies are progressing toward the miniaturization of electronic and electromechanical components, thus augmenting the demand for the developments of high performance conductive materials. In particular, the miniaturization of the aforementioned components requires Cu-based alloys with well-balanced combinations of mechanical strength and electrical conductivity in accordance with their use. Copper–Beryllium (Cu–Be) alloys are one of the most prevalent Cu-based alloys that have been commercially utilized for electromechanical connectors. Moreover, Cu–Be alloys that are properly alloyed and heat-treated by precipitation hardening, can achieve excellent conductive abilities and maximal mechanical strength.1–3) However, the Cu–Be system possesses severe limitations such as toxicity to humans and high production costs. Therefore, the development of novel toxic-free and environmentally-friendly conductive materials, which have high conductive and mechanical abilities comparable to those of Cu–Be alloys is of paramount importance to promote the replacement of Cu–Be alloys globally.
Small additions of Ti to Cu are known to significantly improve the mechanical properties of pure Cu. Cu alloys containing 1–6 at% Ti exhibit a pronounced effect on the precipitation hardening. In particular, above ∼800°C, a single-phase solid solution is formed. However, during isothermal aging below 800°C the solid-solution alloys initially undergo spinodal decomposition, followed by the precipitation of the coherent metastable β′ particles of Cu4Ti with body-centered tetragonal D1a-type ordered structures in the parent grains.4–16) At this stage, the alloys demonstrate maximal yield strengths that are comparable to those of Cu–Be alloys. During prolonged aging, the metastable β′-Cu4Ti particles are gradually replaced by stable β-Cu4Ti ones (orthorhombic structure, a = 0.4523 nm, b = 0.4341 nm, c = 1.2918 nm), which are formed by a grain boundary reaction. The final stage of aging, i.e., the over-aging stage, is characterized by reduced hardening. However, Ti additions pose significant challenges because they cause severe deterioration in the electrical conductivity of pure Cu.17–22) Mg, similar to Ti, is one of the most environmentally-friendly and abundant elements on earth. Therefore, Mg has recently attracted significant attention as a unique alloying element for Cu. Small additions of Mg can enhance various properties of pure Cu due to the solution-hardening effect, with less impact on the electrical conductivity than that in the case of Ti additions.23–26) For example, Cu–Mg supersaturated solid-solution alloys developed by Maki et al.,24,25) and designated as magnesium bronze exhibit favorable strength, stress relaxation resistance and electrical conductivity. Based on the Cu–Mg phase diagram,27) the maximum solid solubility of Mg in Cu is 6.9 at% at 725°C. Further additions of Mg beyond this solubility limit will induce the precipitation of the Cu2Mg phase (cubic, a = 0.7047 nm) as an equilibrium phase. However, several limitations must be addressed to facilitate the advancement of the methods for enhancing the performance of the Cu–Mg alloys to satisfy the increasing demands of automotive and computer technologies.
Herein, we focus on the possible effects of Mg doping on the isothermal aging behaviors of Cu–Ti supersaturated solid-solution alloys. There are limited studies on the observable aging behaviors of ternary Cu–Ti–Mg alloys. Based on binary alloy phase diagrams26) and early studies on binary Cu alloys,16,23,24) the solid solution between Mg and Ti atoms is negligible. Additionally, the activating temperature region for the precipitation hardening of the Cu–Ti solid solution is distinctively higher than the stable temperature region for the Cu–Mg solid solution. Therefore, combined additions of Mg and Ti to Cu in various compositions are expected to promote microstructure control. This microstructure control aims to combine the high strength and conductivity properties by optimizing the balance between precipitation hardening stimulated by Ti solutes and solution hardening by Mg solutes. In this study, several composition types of Cu–Ti–Mg alloys were selected for a comparative study; then, the evolution of the microstructure and the electrical and mechanical properties was investigated. This paper is concentrated on the description of microstructural features observable by advanced microscopy and microanalytical techniques such as high-angle annular detector darkfield scanning transmission electron microscopy (HAADF-STEM) and energy dispersive X-ray spectroscopy (EDS). In addition, we observed some challenging results of the structural investigation obtained using a combined technique of an aberration-corrected HAADF-STEM imaging and atomic-resolution EDS mapping analysis. Based on these observations and analyses, the possible effects of Mg doping on the precipitation behaviors of Cu–Ti alloys and on their mechanical and conductive properties will be elucidated. Moreover, the microstructural features modified by Mg doping, which may result in relatively higher mechanical capabilities, will also be discussed.
Figure 1(a) and 1(b) are Cu-rich sections of the binary phase diagrams of Cu–Ti and Cu–Mg, respectively, and Fig. 1(c) is a Cu-rich portion of the Cu–Ti–Mg ternary phase diagram displayed in the 700°C isothermal section. From Fig. 1, all five alloys are each expected to form a single-phase alloy of the supersaturated solid solution after undergoing solution treatment at temperatures >700°C. However, upon aging at a temperature of ∼450°C, the alloys subsequently exhibited two-phase microstructures consisting of the α-Cu solid-solution and the metastable β′- or the stable β-Cu4Ti precipitates. Table 1 shows the nominal and analysed compositions of the master alloys. We prepared four ternary alloys and one binary alloy with nominal compositions (Table 1). The master alloys with selected nominal compositions were each prepared from a mixture of high-purity metals of Cu (99.99%), Ti (99.99%) and Mg (99.99%) by induction heating under an Ar gas in a graphite crucible (as-cast alloys). The actual compositions of the as-cast alloys were chemically analyzed by inductively coupled plasma mass spectroscopy. From the analyses, the Mg content in each alloy is lower than the corresponding nominal value, which may be attributable to the potential excessive vaporization of Mg from the melts during induction heating. The alloys prepared in this study are designated as Cu–2Ti–1Mg, Cu–2Ti–2Mg, Cu–4Ti–1Mg, Cu–4Ti–2Mg, and Cu–4Ti, which indicate the values of the analysed component rounded to the nearest integers, as shown in Table 1. The as-cast alloys were homogenized at an elevated temperature set between 730 and 950°C to obtain a single phase of α-Cu supersaturated solid solutions (Fig. 2). Then, they were cold-rolled to a thickness of 1 mm (∼90% reduction ratio, 0.25 mm per cold-rolling pass reduction ratio). The alloys were subjected to solid solutioning at 850°C for 0.5 hours, followed by water-quenching (SS specimens). The specimens with a size of 3 mm × 3 mm × 1 mm, were cut from the alloy with their largest surfaces normal to the rolling direction, then isothermally aged at 450°C for various periods from 1–100 h. This aging temperature (450°C) is equivalent to levels within the activating temperature range for the precipitation hardening of the Cu–Ti solid solution and the stable temperature range for the Cu–Mg solid-solution. Therefore, both the precipitation and solution-hardening effects due to the Cu–Ti and Cu–Mg solid solutions, respectively, are likely to be simultaneously activated during aging.



Specimen processing method for all the as-cast alloys.
The microstructural evolutions of the specimens with various compositions during aging were monitored by measuring their electrical conductivity and Vickers hardness. The electrical conductivity was measured at room temperature using a constant DC-current four probe method. The Vickers hardness was evaluated using a micro-Vickers hardness tester (Matsuzawa Co., Ltd) at room temperature and applied load of 0.1 kgf. The average hardness was then obtained from 10 or more indentations. The tensile testing was performed at room temperature using a load testing machine equipped with a video camera (Shimadzu AG-IS) until the specimen fractured. The loading axis in the machine was set parallel to the rolling direction of the specimen and strain rate was set at 1 × 10−3 s−1. Specimens with a cross-section of 5 mm × 1 mm and gauge length of 10 mm were used for the tensile tests.
The microstructures of the SS alloys and aged alloys were investigated using various electron microscopy combined with microanalytical techniques, i.e., SEM, TEM/STEM and EDS. Specimens for the SEM and TEM/STEM analyses were cut from the alloys of interest and thinned by mechanical grinding. They were then electro-polished in a liquid mixture of phosphorous-acid and ethanol controlled at 5°C with a DC voltage application of 10 V and completed by ion milling. The SEM backscattered electron imaging technique was used to examine the microstructures using a field emission scanning electron microscope (JEOL JSM-7800F). Selected area electron diffraction patterns and HAADF-STEM images were taken using a 200-kV electron microscope (JEOL JEM-2100F) and a 300-kV electron microscope (FEI Titan3 G2 60-300 Probe Corrector) equipped with a Super-X EDS system.
Figure 3 shows SEM backscattered electron images obtained from four different SS specimens of Cu–2Ti–1Mg, Cu–2Ti–2Mg, Cu–4Ti–1Mg, and Cu–4Ti–2Mg, which were recorded at low magnification. At first sight, each specimen has a rather similar poly-crystalline microstructure which consists of equiaxed crystal grains with its average size (d) ranging between 40 µm and 60 µm containing many annealing twins. A detailed analysis confirms that these images exhibit an extremely small and dark patchy contrast pattern present in varying degrees throughout the micrographs (e.g., Fig. 3(b) and 3(d)). Based on another experiment using SEM/EDS measurements, these patterns were confirmed to be artifacts of oxide impurities. By considering these findings, we confirmed that each SS specimen consists of a single phase of α-Cu supersaturated solid solution with the respective composition.

SEM backscattered electron images of the SS specimens recorded at low magnification: (a) Cu–2Ti–1Mg; (b) Cu–2Ti–2Mg; (c) Cu–4Ti–1Mg; and (d) Cu–4Ti–2Mg. The d values in each inset are the average grain sizes determined from EBSD analysis.
Figure 4 depicts the Vickers hardness and electrical conductivity of the five different ternary or binary specimens that were subjected to isothermal aging at 450°C for up to 100 h. In Fig. 4, two vertical axes exist on both sides, representing the Vickers hardness (right) and the electrical conductivity (left), against the common horizontal axis representing aging time. Here, the electrical conductivity values were expressed as %IACS, which is the percentage for the electrical conductivity of an international annealed copper standard, 5.81 × 107 Ω/m. In Fig. 4(a), all five specimens exhibit an approximately similar hardening behavior. The Vickers hardness values initially increased sharply as the aging time increased, and subsequently reached broad peaks at 10 h of aging (termed peak aging (PA)), then eventually degraded after a further prolonged aging time. The increase in the Vickers hardness for the Cu–4Ti, Cu–4Ti–1Mg, and Cu–4Ti–2Mg alloys during aging was greater than that for the Cu–2Ti–1Mg and Cu–2Ti–2Mg alloys, thereby suggesting that the age-induced hardening should be primarily determined by the Ti contents. Interestingly, when the hardness capabilities were compared at a fixed aging time for the series of aged alloys, Cu–4Ti, Cu–4Ti–1Mg, and Cu–4Ti–2Mg were ranked higher as the Mg content in the alloy increased. This trend will be discussed later.

Isothermal aging behaviors of the five different ternary or binary SS specimens measured at a test temperature of 450°C, for (a) Vickers hardness and (b) Electrical conductivity.
Regarding the electrical conductivity properties, the measured values of the five SS alloys were varied within a 5–22%IACS range (Fig. 4(b)), which is consistent with the values estimated from Nordheim’s rule, as explained in Appendix. For all five specimens aged at 450°C, the electrical conductivity continued to rise with the aging time until an aging time of 100 h was reached. Remarkably, the conductivity properties show a tendency to vary systematically with strong and weak dependence on the Ti and Mg contents, respectively. Typically, their values decrease considerably with the increase of Ti solutes. The aging behaviors observed for the ternary alloy specimens, i.e., the Vickers hardness or the electrical conductivity property, were not significantly improved when compared to those reported for the binary counterparts without Mg doping. However, Mg doping resulted in an improvement of the mechanical tensile properties.
Figure 5 illustrates typical stress–strain curves observed in the tensile tests conducted at room temperature for the PA specimens with four different compositions, i.e., Cu–2Ti–1Mg, Cu–2Ti–2Mg, Cu–4Ti, and Cu–4Ti–2Mg. The mechanical properties determined from the tests and the SS specimens are shown in Table 2. The comparison of the tensile properties obtained before and after the aging treatment for corresponding pairs revealed that peak-aging treatment, i.e., isothermal annealing at 450°C for 10 h, had considerable effects on the tensile properties, thereby increasing the tensile strength and decreasing the fracture elongation. Figure 5 depicts the general trend that an increase of Ti additions stimulates the tensile strengths more than Mg additions, whereas the opposite is true for the fracture elongation properties. The Cu–4Ti–2Mg specimen, which is the highest-concentration alloy, exhibits the highest performance from the viewpoint of both tensile strength and fracture elongation. Thus, it is worth emphasizing that an adequate amount of Mg doping, e.g., ∼1–2 at%, can significantly enhance the fracture elongation capabilities of the Cu–Ti alloys.

Nominal stress–strain curves measured for the four PA specimens with different compositions.

Figure 6 illustrates SEM backscattered electron images obtained from three different over-aged specimens of Cu–4Ti, Cu–4Ti–1Mg, and Cu–4Ti–2Mg; each of which underwent the entire process of aging (450°C, 100 h). Figure 6(b) depicts an enlarged image of a section of the Cu–4Ti. According to several previous studies,7,8,14) over-aging in binary Cu–Ti age-hardenable alloys is associated with the emergence of characteristic microstructure, which is termed a laminated cellular component. The aforementioned component nucleates and grows discontinuously at the grain boundaries of the parent matrix phase (discontinuous precipitation). The dark contrast regions observed in Figs. 6(a), (c), and (d), are displayed in Fig. 6(b). These regions bear a close resemblance to the characteristic microstructure, i.e., the cellular components, consisting of α-Cu + β-Cu4Ti, as found in the binary over-aged alloys. From Fig. 6, the volume fractions of the cellular components are reduced as the Mg additions are increased. This trend is confirmed by the finding that the area fractions in the dark measured using computer-aided image analysis are 34.4, 13.6, and 8.1% for Cu–4Ti, Cu–4Ti–1Mg, and Cu–4Ti–2Mg, respectively. Thus, it is evident that an increase in the Mg additions results in a reduced generation of the cellular-like microstructure in the vicinities of the grain boundaries present in the ternary over-aged specimens.

SEM backscattered electron images of three different over-aged specimens, each of which was subjected to an isothermal aging treatment at a test temperature of 450°C for up to 100 h: (a), (b) Cu–4Ti; (c) Cu–4Ti–1Mg; and (d) Cu–4Ti–2Mg.
A further metallographic survey of the ternary PA specimens was aimed at identifying unique microstructural features which could account for the higher mechanical performance experimentally demonstrated. Figure 7 shows the SEM backscattered electron images obtained from five different PA specimens: Cu–2Ti–1Mg, Cu–2Ti–2Mg, Cu–4Ti–1Mg, Cu–4Ti–2Mg, and Cu–4Ti, which were recorded at relatively high magnification. Each image was taken by targeting a region containing an individual or a plurality of grain boundaries. Across each boundary, the contrast intensity sharply alternated between bright and dark, which is due to the channeling effect of backscattered electrons. Apart from the electron channeling contrast, each micrograph reveals the microstructural feature responsible for the peak-hardening effect. That is, an abundance of dark patchy contrasts with several tens of nanometers in size and associated with metastable β′-Cu4Ti precipitates are dispersed homogeneously over the entire micrograph. A tendency for the precipitates to lie in rows can also be recognized, similar to the case of the other binary age-hardenable Cu–Ti alloys.4–8) The number densities of the precipitates are higher in the specimens with 4 at%Ti than those with 2 at%Ti. Upon careful inspection of the distributions of the precipitates, precipitate-free zones (PFZs) are observed in the vicinities of grain boundaries, except for twin boundaries, where there are minimal precipitates. The presence of PFZs is most apparent in Fig. 7(a) and, to a lesser extent, in Figs. 7(c) and 7(d). The grain boundary observed in Fig. 7(b), which is not accompanied by any obvious PFZs, is due to the twinning of the matrix crystal. The grain boundary in Fig. 7(e) is also observed to be free from PFZs; however, the vicinities are decorated by another sequence of dark, patchy contrast patterns with slightly larger sizes. These unusual contrast features visible in (e) are associated with discontinuous precipitation, as addressed above.
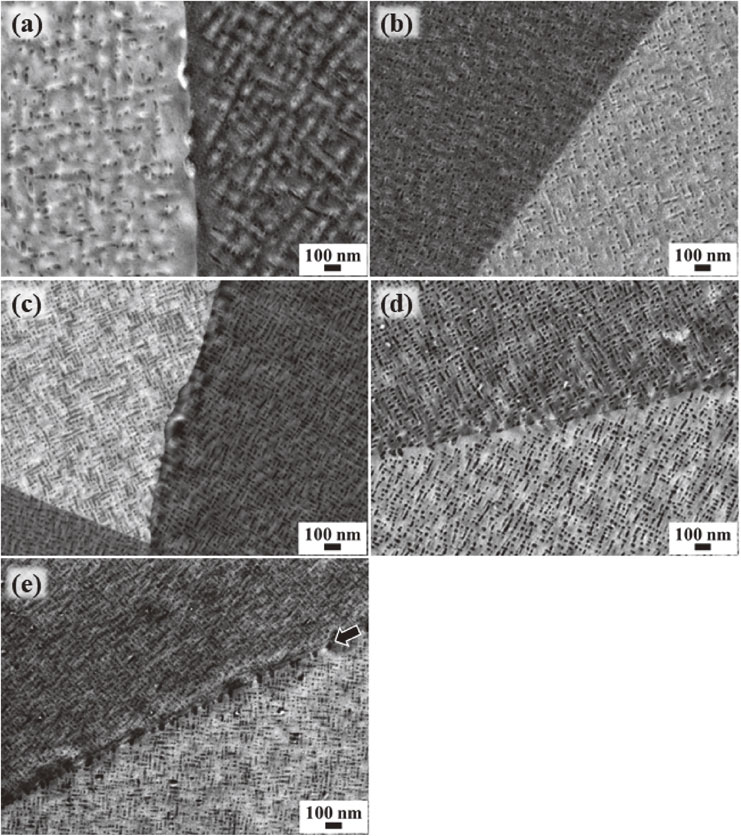
SEM backscattered electron images of the PA specimens recorded at relatively high magnification: (a) Cu–2Ti–1Mg; (b) Cu–2Ti–2Mg; (c) Cu–4Ti–1Mg; (d) Cu–4Ti–2Mg; and (e) Cu–4Ti. In every image, an abundance of dark, patchy contrast patterns associated with specific metastable precipitates are dispersed homogeneously over the entire micrographs with varying degrees of number density. Another sequence of dark, patchy contrast patterns demonstrated slightly larger sizes in the row along the grain boundary in (e), as indicated by the arrow.
Figure 8 illustrates two examples of precipitation microstructures observed by HAADF-STEM for the Cu–2Ti–2Mg and Cu–4Ti–1Mg ternary PA specimens. HAADF-STEM imaging enables us to enhance the contrast features resulting from differences in the atomic numbers of the constituent atoms of the material. Figure 8 depicts the distinct contrast features observed over the entire material that are each considered to represent the metastable precipitate phase responsible for peak hardening. These contrast features display dark, patchy contrast patterns with particle sizes of a few tens of nanometers. Figure 9 depicts a set of STEM/EDS results recorded for the identical microscopic region in the Cu–2Ti–2Mg PA specimen. The HAADF-STEM image is shown in (a) and its corresponding EDS mapping images with the respective constituent elements are displayed separately in (b) Cu–K, (c) Ti–K, and (d) Mg–K. In Fig. 9(a), the slightly round or cuboidal particles displayed in dark contrast correspond to individual regions of patchy contrasts discussed in Fig. 8. The particles are enriched with Ti but are poor in Cu and Mg relative to the surrounding matrix. In other words, the Ti solutes are concentrated in the precipitates, whereas Mg solutes are homogeneously distributed in other regions than in the precipitates. This finding is consistent with the assumption based on the binary alloy phase diagrams being considered: Mg atoms can undergo solid solutioning with Cu, but not Ti, atoms. The EDS spectrum data collected from this micrographic region enabled us to estimate that the cuboidal particles and their surrounding matrix regions have respectively the compositions of Cu81.8Ti16.5Mg1.7 and Cu97.6Ti0.225Mg2.13 on average. We suggest that the solid solution of such a slight but certain amount of Mg affects the stability of the β′-Cu4Ti phase as well as the morphology. In fact, many of the β′-precipitates present in the ternary alloys are seen to take the form of isolated cuboids rather than solid rods as commonly found for the binary alloys.

HAADF-STEM images of the precipitation microstructures formed in the ternary PA specimens, (a) Cu–2Ti–2Mg and (b) Cu–4Ti–1Mg alloys, were recorded at intermediate magnification. The dark, patchy contrast patterns with particle sizes of a few tens of nanometers, are each considered to represent a specific metastable precipitate phase responsible for peak hardening.

Enlarged HAADF-STEM image obtained from the Cu–2Ti–2Mg PA specimen, and the corresponding EDS mapping images of the respective constituent elements: (a) HAADF-STEM image; (b) Cu–K; (c) Ti–K; and (d) Mg–K.
The aberration-corrected HAADF-STEM observation combined with the atomic-resolution EDS mapping analysis was conducted in an attempt to gain new insights on the precipitates responsible for the peak hardening observed in the present ternary specimens. Figure 10(a) depicts the atomic-scale HAADF-STEM image targeted at one of the patchy contrast particles found in the Cu–2Ti–2Mg PA specimen, which was taken in the [001]α incidence of the α-Cu FCC matrix. The subscript letter α attached to the miller index represents the α-Cu matrix; and, the inset (b) shows the digital Fourier diffractogram generated from (a), which was performed using a computational treatment. In Fig. 10(a), the precipitate appears to be cuboid-shaped on a dark background. An extremely small-scale contrast feature existing on the entire micrograph, consisting of bright dots arranged in a square lattice, corresponds to the projected fundamental unit of the crystal structure existing on the spot under investigation. A closer examination confirms that the contrast pattern arising from the precipitate particle is distinct from that arising from the surrounding α-Cu FCC matrix. Moreover, the contrast of the precipitate displays a more intricate pattern with a strong resemblance to a checker-board design. Accordingly, the resulting Fourier diffractogram has two sets of overlapping square lattice patterns with different origins. One type is the fundamental pattern due to the α-Cu FCC structure, in which two of the constituent spots are indicated by 200α and 020α in the inset (b). The other type of lattice pattern generated due to the precipitate consists of weaker spots with shorter interval distances. It is rotated by a certain angle relative to the fundamental lattice around the typical normal axis assumed at a position indicated by 000. All the contrast features of this precipitate depicted by Fig. 10 correlate best with the model structure assumed for the metastable β′-Cu4Ti phase with the body-centred tetragonal D1a-type ordered structure.6–8,14) Under this assumption, the spots generated by the precipitate are designated as 1/5{420} in the reciprocal coordinate based on the fundamental FCC lattice. In Fig. 10(a), the rows of lattice dots lying along the [002]α - (or [020]α -) directions continue smoothly over the interface between the precipitate and the α-Cu matrix, or are slightly bent.

(a) Atomic-scale HAADF-STEM image of a single precipitate particle of the β′ crystal present in the Cu–2Ti–2Mg PA specimen, which was taken in the [001]α incidence of the α-Cu FCC matrix, together with (b) the digital Fourier diffractogram of the lattice image in the inset.
Figure 11(a) illustrates an enlarged atomic-scale HAADF-STEM image from a local region of the β′ particle observed in Fig. 10(a). Figures 11(b)–(d) depict atomic-resolution EDS mapping images displayed separately with the respective constituent elements, i.e., Cu–K, Ti–K, and Mg–K. This combined technique allowed us to distinguish individual atomic columns in the [001]α projection of the FCC-Cu structure modified by the chemical ordering of Cu and Ti. A frame format of the projected unit cell profile for the β′ crystal in the [001]α incidence is inserted in (a), (b), and (c), where the atomic columns enriched with Ti or Cu are indicated in red or green, respectively. In contrast, Mg atoms are randomly substituted in the FCC lattice. Thus, the model structure proposed for the metastable Cu4Ti crystal, which has been widely recognized as the body-centered tetragonal D1a-type ordered structure,6–8,14) can best describe all the details presented by Figs. 9–11.

Enlarged atomic-scale HAADF-STEM image of a local region of the β′-Cu4Ti precipitate shown in Fig. 10, and the corresponding EDS mapping images of the respective constituent elements: (a) HAADF-STEM image; (b) Cu–K; (c) Ti–K; and (d) Mg–K. The chemical ordering of Cu and Ti occurs in the FCC-Cu lattice.
Based on these observations, we discussed several existing relationships between the various mechanical/conductive properties and characteristic microstructural features observed by the Cu–Ti–Mg age-hardenable alloys. Present studies on the isothermal aging behaviors of ternary supersaturated solid-solution alloys reveal that their precipitation-hardening effect is due to the generation of the metastable β′-Cu4Ti phase in the α-Cu matrix, which is similar to the cause of the effect in the Cu–Ti binary system. For example, the Cu–4Ti–2Mg alloy PA specimen demonstrates outstanding mechanical performance, yielding high levels of Vickers hardness, tensile strength, and tensile fracture elongation, with a slightly lower electrical conductivity than that achievable by the binary counterpart without Mg doping. We observed an abundance of fine precipitates involving the chemical ordering of Cu and Ti in the α-Cu matrix of all the different composition types of PA specimens. Also, we noticed that a large part of the Mg solutes is homogeneously distributed over the matrix regions, while there is also a smaller part of them present in the precipitates. (the STEM/EDS quantitative analysis aimed at seeking more accurate information is currently ongoing.) An assumption was made that two types of solute atoms, i.e., Ti and Mg, could independently contribute to the solution-hardening effect for Cu with different rates. The assumption only holds for the rates of approximately 9 and 5 HV/at% for the Ti22) and Mg23) solutes, respectively. Hence, it later became apparent that the Vickers hardness values observed particularly for the SS specimens are compatible with the estimated values under the assumption above. A similar observation is noted for their tensile strength properties due to the positive correlation between hardness and tensile strength. The ternary specimens exhibited improved electrical conductivities (5–22%IACS) during aging (until an aging time of 100 h). However, there was a general tendency for their conductivity values to decrease strongly or weakly with an increase in Ti or Mg solutes, respectively. These observations strongly agree with the estimated results from Nordheim’s rule30) (see Appendix). In summary, various observable mechanical/conductive properties for Cu–Ti–Mg age-hardenable alloys are approximated from the sum of the precipitation-hardening effect due to Ti solutes and the solid-solution hardening effect due to both Ti and Mg solutes.
The structural environment that is the determining factor in the Cu–Ti–Mg alloys resulting in excellent fracture elongation properties (Fig. 5) is still not elucidated. From the information known, the dark contrast regions observed in Figs. 6(a), (c), and (d) are identified as laminated cellular components containing α-Cu + β-Cu4Ti, which are regarded as the typical microstructures of over-aged alloys with the Cu–Ti binary system. In general, the growth of cellular components steadily advances, replacing the metastable β′-Cu4Ti precipitate with the stable β-Cu4Ti precipitate with an orthorhombic structure (prototype: Au4Zr; space group: Pnma). This replacement leads to rapid degradation of the mechanical properties.7,8,14) Hence, cellular components are detrimental to the attainable tensile capability. From Fig. 6, it can be deduced that addition of as much as ∼1–2 at% Mg substantially inhibits discontinuous precipitation of the cellular components from occurring along the grain boundaries. Thus, the progression of over-aging represented by the distribution of the stable β-Cu4Ti precipitates is delayed. One could then interpret that the volume reduction of cellular components observed resulted from the solid solution of Mg atoms in the α-Cu matrix. From Fig. 7, there are PFZs distributed in several vicinities of the grain boundaries present in the ternary PA specimens. Figure 7(e) indicates that the Cu–4Ti PA specimen, which is not influenced by Mg solutes, had discontinuous precipitation sooner than 10 h after aging began. Thus, it is postulated that the grain boundaries in ternary alloys can remain structurally intact during prolonged aging and do not undergo discontinuous precipitation due to the presence of Mg solutes. The explanations above may account for the high mechanical properties of the Cu–Ti–Mg age-hardenable alloys. Thus, the microstructures of Cu-based alloys containing adequate amounts of Ti and Mg solutes (4 at% Ti, 1.6 at% Mg) are capable of yielding combined properties of high-strength and high-fracture elongation. However, the aforementioned can only be achieved when the alloys undergo suitable solid solutioning and subsequent aging at an elevated temperature of ∼450°C for 10 h. Moreover, both the effects of precipitation-hardening by Ti and solution-hardening by Ti and Mg should act synergistically.
Isothermal aging behaviors of Cu–Ti–Mg supersaturated solid-solution alloys with different compositions have been thoroughly examined in a comparative study using various electron microscopy and microanalytical techniques. The tests were conducted at a temperature of 450°C and an aging time of up to 100 h. The major findings are summarized as follows:
This work was performed under the inter-university cooperative research program (Proposal No. 19G0004) of the Cooperative Research and Development Center for Advanced Materials, Institute for materials Research, Tohoku University. A part of this work was supported by Tohoku University, Microstructural Characterization Platform in Nanotechnology Platform Project sponsored by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. It was also supported by Japan Copper and Brass Association foundation in 2019. This research was funded by the Japan Society for the Promotion of Science (JSPS) as a Grant-in-Aid for Scientific Research (B) (No. 18H01743).
The electrical resistivity of the metallic binary solid solution, ρss, is expressed in the analytic form proposed by Nordheim. Using the most simplified case assumed for dilute alloys, the increase in the electrical resistivity, Δρss (Ω·m), of the solid solution is given by the following equation:30)
| \begin{equation} \Delta\rho _{\text{ss}} = A_{\text{i}}C_{\text{i}}(1 - B_{\text{i}}C_{\text{i}}) \approx A_{\text{i}}C_{\text{i}}, \end{equation} | (A.1) |