2020 Volume 61 Issue 11 Pages 2095-2100
2020 Volume 61 Issue 11 Pages 2095-2100
Recently, the remarkable strengthening effect of α-Al(Mn, Fe) Si dispersoids at both ambient temperature and elevated temperature were found by several researches. In AA3xxx alloys, a large amount of dispersoids can form by applying suitable heat-treatment. In the present work, the influences of Cr addition on mechanical properties and microstructures of the Al–Mn–Mg–Si alloys were investigated. The mechanical properties at ambient temperature were evaluated by micro-hardness and yield strength. Yield strength at 300°C and creep resistance at 300°C were used to evaluate materials’ mechanical properties at elevated temperature. Moreover, the microstructures in as-cast and heat-treated conditions were quantitatively analyzed by optical and transmission electron microscopes. Results revealed that the addition of Cr increased area percentage of Mn-containing intermetallic particles. It also indicated that solubility of Mn element decreased due to Cr addition. Very little amount of Cr were detected in Mn-containing intermetallic particles and dispersoids. The distribution of dispersoids was not influenced by Cr addition. Number density and volume fraction of dispersoids decreased because of Cr. Electrical conductivity decreased significantly because of 0.30% Cr addition which indicated that a large amount of Cr were still in solid solution condition. Micro-hardness and yield strength at ambient temperature increased with the increasing content of Cr, and the Cr addition increased yield strength at elevated temperature as well. Moreover, creep resistance at 300°C improved dramatically with the increasing content of Cr.
Addition of Cr enhanced material’s creep resistance in form of solid solution rather than dispersoid.
Traditionally, AA3xxx aluminum alloys were widely used in packaging, automobile and architecture industries, because of its good formability, excellent corrosion resistance and weldability. And AA3xxx alloys were considered as non-heat-treatable alloys. However, the strengthening effect of dispersoids in AA3xxx alloys was discovered recently.1–4) A large amount of dispersoids could precipitate by a proper heat treatment.
In order to further improve mechanical properties of AA3xxx alloys, the composition of the alloys should be further optimized. A few previous studies reported the influence of compositions on AA3xxx alloys. Adding Mn element could enhance the precipitation of α-Al(Mn, Fe)Si dispersoids and improve yield strength,5–7) as long as the concentration of Mn was under the solid solution limit. Fe decreased the solubility of Mn and accelerated the precipitation rate of α-Al(Mn, Fe)Si dispersoids,2) the yield strength and creep resistance at elevated temperature could increase with an optimized content of Fe.8) Mg element would affect nucleation process of α-Al(Mn, Fe)Si dispersoids by forming metastable Mg2Si,9–11) metastable Mg2Si precipitated during heating process and promoted the precipitation of α-Al(Mn, Fe)Si dispersoids,11) moreover, Mg provided solid solution strength for AA3004 alloys.4) Si increased the volume fraction of α-Al(Mn, Fe)Si dispersoids5) and decreased the size of α-Al(Mn, Fe)Si dispersoids.12) Cu decreased the size of dispersoids and improved dispersoids’ thermal stability.13) The addition of Sc and Zr could greatly increase yield strength at room temperature and creep resistance at elevated temperature by introducing Al3(Sc, Zr) into AA3xxx alloys.14) With the addition of Mo, the size of dispersoids became finer with a higher volume fraction, the area percentage of dispersoid free zones was reduced. Elevated temperature yield strength and creep resistance improved due to Mo addition.15,16) However, very few researches about the effect of Cr elements on the microstructure and mechanical properties of AA3xxx alloys were reported.
In the present study, the effect of Cr on microstructure and mechanical properties at room temperature and elevated temperature of Al–Mn–Mg–Si alloys were investigated. The microstructures are quantitatively evaluated by optical microscope and transmission electron microscope. The micro-hardness and yield strength at ambient temperature were measured and creep tests and yield strength at elevated temperature were also conducted.
Two alloys were designed to study the effect of Cr. The chemical compositions of these alloys were shown in Table 1. The content of Cr changed from 0% to 0.28%. DC0 and DC30 alloys contained 0% and 0.28% Cr respectively. These alloys were prepared by electric resistance furnace. The melt were maintained at 750°C for 30 minutes and degassed for 15 minutes. Then the melt were solidified in a permanent steel mold preheated at 250°C. The dimension of the ingot was 30 mm × 40 mm × 80 mm. In order to precipitate α-Al(MnFe)Si dispersoids, the samples were heated to 375°C with a 5°C/min heating rate. The holding time were from 2 h to 24 h. Afterwards, the samples were water quenched to room temperature.

Vicker hardness were measured with a 200 g load and a 20 s dwelling time. 10 measurements were performed at the polished samples to obtain the average value of each sample. Compression yield strength tests were conducted at 25°C and 300°C by a Gleeble 3800 thermomechanical simulator unit with a strain rate of 0.001 s−1. The Gleeble samples were machined into a cylinder form with 15 mm in length and 10 mm in diameter. Three measurements were conducted to get the average values. Compression creep tests were performed at 300°C for 96 h with 58 MPa load. Each creep test was repeated twice. The creep samples were the same size as the Gleeble samples.
Optical microscope and scanning electron microscope were used to observe the as-cast and heat-treated microstructures. In order to reveal the distribution of dispersoids clearly, the polished samples were etched by 0.5% HF for 25 seconds. The area fraction of intermetallic particles and dispersoid free zone (DFZ) were quantified by image analysis software (Clemex PE 4.0) with polished and etched samples respectively. TEM foils were prepared by twin-jet machine using a solution of 30% nitric acid in methanol at −25°C. Transmission electron microscope (TEM, JEM-2100) operated at 200 kV was used to observe the precipitation behavior dispersoids. Incident beam was along ⟨100⟩ zone axis and {200} planes were recorded in two beam conditions. An electron energy loss spectroscope (EELS) attached to the TEM was used to measure the thickness of the samples. The volume fraction of dispersoids was calculated according to the method introduced in previous literature2) shown in eq. (1):
| \begin{equation} V_{v} = A_{A}\frac{K\bar{D}}{K\bar{D} + t}(1 - A_{\textit{DFZ}}) \end{equation} | (1) |
Figure 1(a) and (b) were the as-cast microstructure of DC0 and DC30 alloys. Two types of intermetallic particles presented in the alloys. The black particles were primary Mg2Si and the grey color particles were Mn-containing intermetallics. The morphologies were consistent with previous literature.17–19) For base alloys, DC0 alloys, the grey color phases were Al6(Mn, Fe) intermetallic particles. On the other hand, Cr elements were detected in the grey phases of DC30 alloys according to EDS results as shown in Fig. 2. These phases were identified as Al6(Mn, Fe, Cr) intermetallics based on the chemical composition and morphology. About 20 EDS spectrum measurements were conducted on Al6(Mn, Fe, Cr) intermetallics. The average ratio of Mn/Cr and Fe/Cr of Al6(Mn, Fe, Cr) intermetallics were 11.98 and 14.23 respectively. The calculated results were shown in Table 2. So Al6(Mn, Fe, Cr) intermetallics could be identified as Al6(Mn0.44Fe0.52Cr0.04) in the present study. Based on the results above, only trace amount of Cr was detected in the intermetallic particles. But the addition of Cr increased the amount of Mn-containing intermetallic dramatically. The area fraction of Mn-containing intermetallic particles and primary Mg2Si were measured by image analysis software. The quantitative results were shown in Table 3. The area fraction of Mn-containing intermetallic particles in DC0 alloys (0%Cr) was 3.67%, however, in DC30 alloys (0.28%Cr) the area fraction increased to 4.54%, even though very little amount of Cr participated the formation of Mn-containing intermetallics. It indicated that Cr addition decreased the solubility of Mn in aluminum alloys which caused the area fraction increment of Mn-containing intermetallics. On the other hand, the amount of primary Mg2Si were not affected by the addition of Cr elements. The area fraction of primary Mg2Si were 0.11% and 0.12% for DC0 alloys (0%Cr) and DC30 alloys (0.28%Cr) respectively.

As-cast microstructure of (a) DC0 alloys and (b) DC30 alloys.
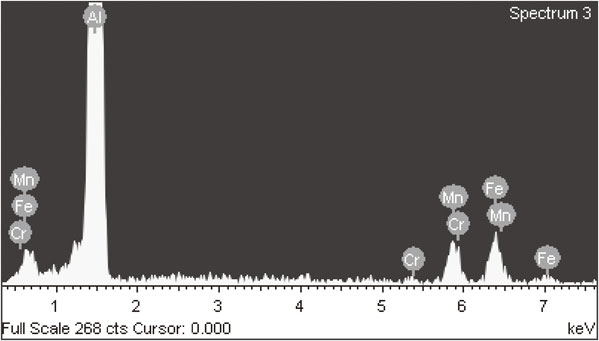
The EDS spectrum of Mn-containing intermetallic particles in DC30 alloys.


After heat-treatment at 375°C for 24 h, a large amount of dispersoids would precipitate in AA3xxx alloys, which had been confirmed in the previous researches.11,14) But due to the small size of dispersoids, they could not be observed by optical microscope. In order to observe the distribution of dispersoids, the alloys were etched by 0.5%HF for 25 s. Then three types of microstructure could be observed as shown in Fig. 3(a) and (b). The black particles were intermetallic particles which had been etched. The areas with darker color were defined as dispersoid zone. Within dispersoid zone, large number density of dispersoids were found according to TEM observation of previous literature.2,3,12) On the other hand, the light color areas were defined as dispersoid free zone (DFZ). This was due to low number density of dispersoids inside dispersoid free zone. By analyzing dispersoid zone and dispersoid free zone, the distribution of dispersoids in large scale could be evaluated. In the present study, the distribution of dispersoid free zone and dispersoid zone were very similar for DC0 and DC30 alloys. Dispersoid free zone presented around intermetallic particles and along grain boundaries where Mn and Cr elements tended to be depleted. The segregation of Mn and Cr elements resulted in the sparse precipitation of dispersoids. And the area fraction of dispersoid zone and dispersoid free zone were quantified, the results were shown in Table 4, the area fraction of dispersoid zone slightly decreased from 69.69% to 66.59% due to the addition of Cr element. It indicated that Cr had negligible effects on the distribution of dispersoids.
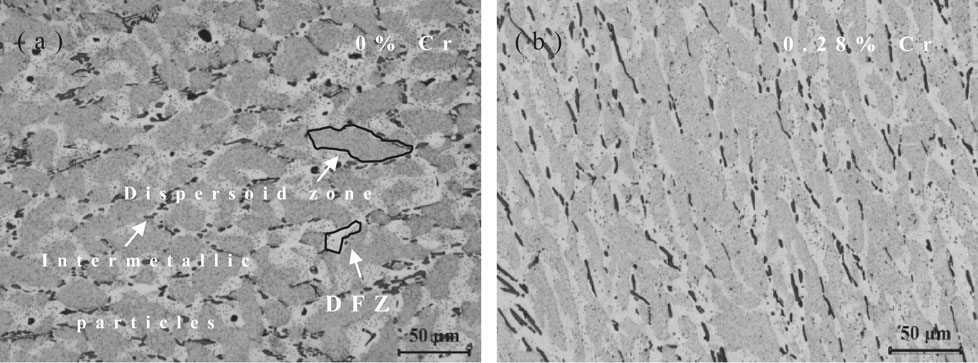
After heat-treatment at 375°C for 24 h, dispersoid zone and dispersoid free zone of (a) DC0 alloys and (b) DC30 alloys.

In order to observe the morphology of dispersoids, transmission electron microscope (TEM) had to be used due to the small size of dispersoids. After heat-treatment at 375°C for 24 h, a large amount of dispersoids precipitated in both DC0 alloys and DC30 alloys as shown in Fig. 4(a) and (b). The morphology of the dispersoids in these two alloys was quite similar, which was also consistent with previous literature.4,20) Plate-like and rod-like dispersoids were found, actually they were two dimensions projections of three dimensions morphology. According to previous literature,20) the true 3D morphology of dispersoids were disc-shaped.

After heat-treatment at 375°C for 24 h, the morphology of dispersoids in (a) DC0 alloys and (b) DC30 alloys.
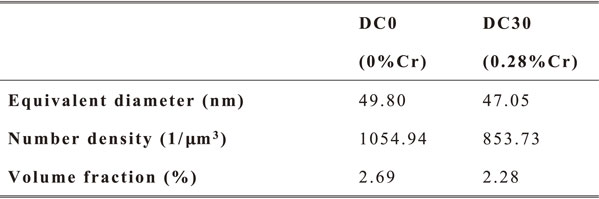
In Cr-free DC0 alloys, the dispersoids could be identified as α-Al(Mn, Fe) Si dispersoids based on the shape and composition, which agreed with many previous works.1–3,5,8) However, in DC30 alloys, a small amount of Cr element were detected in the dispersoids according to the EDS spectrum results as shown in Fig. 5. So the dispersoids in DC30 alloys could be identified as α-Al(Mn, Fe, Cr) Si dispersoids. The atomic ratio of Mn/Cr of α-Al(Mn, Fe, Cr) Si dispersoids in DC30 alloys varied with each dispersoids, but generally the amount of Cr were much lower than Mn. The amount of Fe was very few in the dispersoids of both DC0 and DC30 alloys. This was due to the extremely low solubility of Fe in aluminum. Most of the Fe participated in forming Al6(Mn, Fe) or Al6(Mn, Fe, Cr) intermetallic particles during solidification. As shown in Table 5, the equivalent diameter, number density and volume fraction of dispersoids were quantitatively analyzed based on TEM images. The equivalent diameter of dispersoids in DC0 alloys and DC30 alloys were 49.80 nm and 47.05 nm respectively. The size of dispersoids slightly decreased because of the addition of Cr. But the number density of dispersoids dropped dramatically from 1054.94 µm−3 to 853.73 µm−3 caused by Cr addition. As a result, the decrement of number density leaded to the loss of volume fraction of dispersoids. The volume fraction of dispersoids of DC0 alloys and DC30 alloys were respectively 2.69% and 2.28%. DC30 alloys possessed less dispersoids than DC0 alloys. This may be due to the area fraction increment of Mn-containing intermetallic particles in DC30 alloys. The area fraction of Mn-containing intermetallic particles in as-cast microstructure, increased from 3.67% to 4.54% because of Cr addition, as shown in Table 3. It indicated that more amount of Mn elements was consumed by Mn-containing intermetallic particles during casting process. Moreover, these intermetallic particles were insoluble at 375°C. Therefore, the available content of solute Mn element for the precipitation of dispersoids decreased, meanwhile very limited amount of Cr participated the precipitation of dispersoids, as a result, the overall volume fraction of dispersoids decreased because of Cr addition.

The EDS spectrum of α-Al(Mn, Fe, Cr) Si dispersoids precipitated in DC30 alloys.
Electrical conductivity of DC0 alloys and DC30 alloys were measured to evaluate the solute element content in aluminum matrix. The results were shown in Fig. 6(a). While the heat-treatment holding time increased, the electrical conductivity of DC0 alloys and DC30 alloys first climbed sharply then increased slowly. This was caused by the precipitation of dispersoids. The precipitation of dispersoids leaded to the loss of Mn, Cr and Si elements solute in aluminum matrix. So, the precipitation rate of dispersoids could be roughly indicated by the electrical conductivity increment rate. The dispersoids firstly precipitated rapidly and then the precipitation became slower. On the other hand, the electrical conductivity of DC30 alloys was lower than DC0 alloys. It illustrated that the amount of solute elements in DC30 alloys were higher than in DC0 alloys. Based on the EDS spectrum results and Mn/Cr ratio, only a small amount of Cr element found in the intermetallic particles and dispersoids. It indicated that still a large amount of Cr element were in solid solution.

(a) Electrical conductivity and (b) Micro-hardness as a function of holding time at 375°C.
Figure 6(b) was the results of micro-hardness after holding at 375°C from 2 h to 48 h. For both DC0 alloys and DC30 alloys, the micro-hardness evolution curves were very similar. At first, the hardness kept increasing sharply until the holding time reached 12 h. Then, as the holding time increased, the hardness increased slightly. As the heat-treatment holding time increased, the precipitation of dispersoids continued which resulted in the hardness increment. Just like electrical conductivity curves, the micro-hardness curve also represented the precipitation behavior of dispersoids. So the tendency of micro-hardness curves (Fig. 6(b)) were highly consistent with electrical conductivity curves (Fig. 6(a)). Moreover, DC30 alloys which were with 0.3% Cr addition had a higher hardness than Cr-free DC0 alloys. But the volume fraction and number density of dispersoids in DC30 alloys were even lower than in DC0 alloys. This could be contributed to the solid solution strengthening of Cr. According to the discussion above, very little Cr formed intermetallic particles or dispersoids, so still a lot of Cr elements were in aluminum matrix and provided strength by solid solution strengthening.
3.5 Yield strength at ambient and elevated temperature and creep resistance at elevated temperatureAfter heat-treatment at 375°C for 24 h, the yield strength of DC0 alloys and DC30 alloys were measured at both 25°C and 300°C. The results were respectively shown in Fig. 7(a) and (b). The yield strength measured at room temperature of DC0 alloys and DC30 alloys were 97.5 MPa and 105.8 MPa. On the other hand, the yield strength measured at 300°C for DC0 alloys and DC30 alloys were 81.2 MPa and 82.8 MPa. With 0.3% Cr addition, the yield strength measured at 25°C and 300°C increased 8.3 MPa and 1.6 MPa respectively. Although materials’ strength at room and elevated temperature both increased because of Cr addition, the strengthening effect was very limited. It was well known that dispersoids were the key strengthening phases for AA3xxx alloys at both room and elevated temperature.3,5,12,21) But in the present study, the volume fraction of dispersoids decreased due to Cr addition (Table 5). However, the room and elevated temperature strength of materials increased as Cr content increased. It indicated that the strengthening effect of Cr for present study was not dispersoid strengthening. On the other hand, very small amount of Cr element found in the intermetallic particles and dispersoids. It means that most of Cr element were still in solid solution. Therefore, Cr addition mainly strengthened materials by solid solution strengthening.
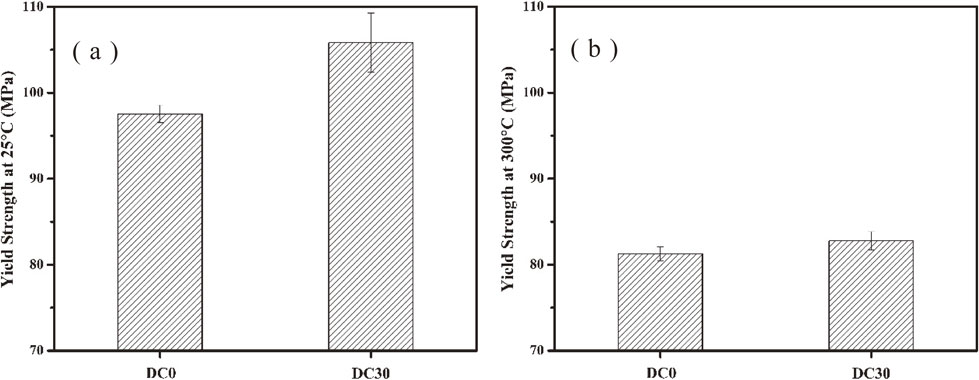
After heat-treatment at 375°C for 24 h, compression yield strength results (strain rate 0.001 s−1) of DC0 alloys and DC30 alloys at (a) 25°C and (b) at 375°C.
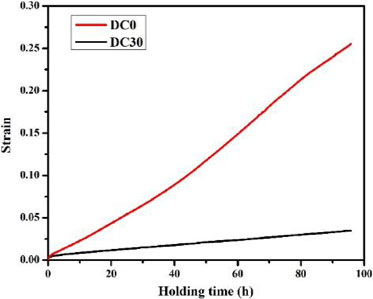
Moreover, in order to evaluate materials’ elevated temperature mechanical properties, compression creep resistance tests were also conducted at 300°C. The creep curves were shown in Fig. 8. After holding at 300°C with 58 MPa load for 96 h, the strain of DC0 alloys and DC30 alloys were 0.25 and 0.025. Materials’ creep resistance at elevated temperature improved dramatically because of 0.3% Cr addition. Solute Cr atoms caused the lattice distortion and stress field. They restricted the movements of dislocations. Owing to the strong attractive force between solute Cr atoms and dislocations, an extra external stress was needed before the dislocations break away. According to the discussions above, the key influence of Cr addition on mechanical properties was solid solution strengthening. Even though the effect of Cr on yield strength at both room temperature and elevated temperature were limited, the creep resistance of AA3xxx alloys improved greatly due to solid solution strengthening effect of Cr. For future alloy development which requires creep resistance properties at elevated temperature, Cr addition should be an excellent choice.

After heat-treatment at 375°C for 24 h, the creep curve measured at 300°C for 96 h with 58 MPa load of DC0 and DC30 alloys.
The authors would like to acknowledge the financial support of National Natural Science Foundation of China (Grant No. U1864209) and China Postdoctoral Foundation Grant (Grant No. 2018M642309).