2020 Volume 61 Issue 12 Pages 2419-2427
2020 Volume 61 Issue 12 Pages 2419-2427
Disks of ferrous and titanium-based material were immersed and unidirectionally slid against a stainless steel pin in ethanol with or without acetic acid to evaluate the effect of acetic acid on wear. The disks were made of cast iron, stainless steel, titanium metal and particulate-reinforced titanium metal matrix composite sintered from mixed powder of Si3N4 (5 mass%), TiN (10 mass%) and Ti (85 mass%). In the absence of sliding, the stainless steel, titanium and composite showed good corrosion resistance but the cast iron exhibited galvanic corrosion. With sliding, the addition of acetic acid to the ethanol increased the wear of the ferrous materials but had little effect on the titanium-based materials. Morphological and chemical analyses of the worn surfaces revealed that acetic acid promoted anodic dissolution of iron on the sliding surfaces of the ferrous materials in ethanol. The results also indicated that titanium and the composite in ethanol with and without acetic acid primary underwent mechanical wear and chemical wear, respectively. The composite exhibited good corrosion and wear resistance in ethanol regardless of the presence of acetic acid.

Fig. 6 Specific wear rates of disks and pins: (a) disk and (b) SUS440C stainless steel pin. The symbols “*” and “**” indicate that the wear could not be calculated.
The purpose of this study was to develop wear-resistant materials for use with fuel based on renewable ethanol derived from plant biomass. At present, ferrous materials such as cast iron and carbon steel are typically employed in petroleum fuel pipelines and storage tanks. However, corrosive impurities such as sodium chloride and acetic acid can be found in fuel-grade biomass ethanol, the latter of which was the focus of this research.1–3) Many other groups have previously investigated the corrosive effects of acetic acid on ferrous materials immersed in fuel grade ethanol.1,4–8)
In solution, acetic acid can dissociate into acetate anions and protons (Scheme (1)).9)
| \begin{equation*} \text{CH$_{3}$COOH} \leftrightarrows \text{CH$_{3}$COO$^{-}$} + \text{H$^{+}$}\quad \text{Scheme (1)$^{9)}$} \end{equation*} |
| \begin{align*} \text{2CH$_{3}$COOH} + \text{Fe}&\to \text{2CH$_{3}$COO$^{-}$} + \text{2H$^{+}$} + \text{Fe$^{2+}$} + \text{2e$^{-}$}\\ &\to \text{(CH$_{3}$COO)$_{2}$Fe} + \text{H$_{2}$} \quad \text{Scheme (2)$^{1)}$} \end{align*} |
The equipment that used to transport biomass ethanol fuel incorporates moving parts such as pistons and cylinders, and the materials of which these are made must be resistant to corrosion and wear when in contact with the fuel. However, there has been little research focusing on the wear resistance of materials in ethanol containing corrosive impurities such as acetic acid. Therefore, the present work investigated the wear behavior of various materials in ethanol with and without acetic acid.
Stainless steel and titanium metal are known to show a high degree of resistance to corrosion by acetic acid.12) A chromium oxide (Cr2O3) rich-iron oxide (Fe2O3)–Cr2O3 film forms on the surface of stainless steel, while titanium oxide (TiO2) film forms on the surface of titanium, both of which are excellent passivation layers.13–17) However, the wear resistance of titanium and its alloys is poor.18,19) It is often possible to improve the wear resistance of such materials by the addition of particulate reinforcements.20–30) As an example, our own group previously reported that the wear resistance of titanium metal matrix composites produced by sintering a mixture of Si3N4, TiN and Ti powders was much higher than that of titanium metal.28,29) On this basis, we considered that a particulate-reinforced titanium metal matrix composite could perform well during sliding tests in biomass-based ethanol.
In the present work, immersion and sliding tests were performed using cast iron, stainless steel, titanium metal and particulate-reinforced titanium metal matrix composite specimens in ethanol both with and without acetic acid, to evaluate wear resistance. The effect of acetic acid on the wear of the test materials are discussed herein based on the analysis of specific wear rates as well as the morphologies and chemical compositions of the specimen surfaces.
The test specimens were disks (diameter: 20 mm, thickness: 5 mm) made of FC250 cast iron, SUS440C stainless steel, titanium metal (purity >99.7%) and particulate-reinforced titanium metal matrix composite and hemispherical pin (radius: 2 mm) made of SUS440C stainless steel. The particulate-reinforced titanium metal matrix composite disks were prepared from a powder mixture comprising 5 mass% Si3N4, 10 mass% TiN and 85 mass% Ti by spark plasma sintering at 1573 K.30) Hereafter, this material is referred to as the 5-10-85 composite. The surfaces of the 5-10-85 composite disks were polished using a no. 1000 grade diamond-grinding wheel, and chemical analysis of the 5-10-85 composite was performed by X-ray photoelectron spectroscopy (XPS, Theta Probe XPS system, Thermo Fisher Scientific). The physical properties of the materials and the chemical composition of the ferrous materials are summarized in Tables 1 and 2, respectively.31–33) The lubricants employed were dehydrated ethanol (C2H5OH, 99.5%, H2O < 50 ppm) and a 5 vol% solution of acetic acid in ethanol. To accelerate corrosion of the tested materials, the concentration of acetic acid in the lubricant was much higher than that in fuel-grade biomass ethanol.1–3)


To evaluate the effect of adding acetic acid to the ethanol on the morphologies and chemical composition of the specimen surfaces under the condition not involving sliding, immersion tests were performed. In the immersion test, disks of the FC250 cast iron, SUS440C stainless steel, titanium metal and 5-10-85 composite were immersed in ethanol with and without acetic acid for approximately 7 h at room temperature, after which the disks were rinsed with ethanol and dried in air.
2.3 Sliding testSliding tests were performed using a unidirectional pin-on-disk machine (FPR-2100, RHESCA), which has been described elsewhere.34) Disks of FC250 cast iron, SUS440C stainless steel, titanium metal and the 5-10-85 composite were slid against SUS440C stainless steel hemispherical pins in ethanol with and without acetic acid with an applied load of 0.49 N, sliding speed of 40 mm/s, sliding time of 100 min and sliding distance of 240 m. The initial mean Hertzian contact pressures between the stainless steel pin and the cast iron, stainless steel and titanium metal disks were 0.35, 0.45 and 0.36 GPa, respectively. The initial mean Hertzian contact pressure between the stainless steel pin and the 5-10-85 composite disk was 0.42 GPa, assuming that the Poisson’s ratio of the 5-10-85 composite was 0.3. The sliding tests were performed with high PV (pressure-velocity) values (14∼18 MPa·m/s) to accelerate wear. Each test was performed three times. After the sliding test, the disk and pin were rinsed with ethanol and then dried in air.
The wear volume of the pin (V(pin)) was calculated from the wear diameter using the eq. (1)35)
| \begin{equation} V(\textit{pin}) = \pi d^{4}/(64r), \end{equation} | (1) |
The surface roughness (Ra) of each immersed disk and cross-sections of the wear track on the disk were examined using a stylus-type surface texture and contour measurement instrument (SURFCOM 1500, TOKYO SEIMITSU). Three-dimensional (3D) profiles of the sliding surfaces of the pins and the disks were acquired with a laser microscope (VK-9500, KEYENCE). The morphologies and chemical compositions of the surfaces of disks and pins were assessed by tungsten filament scanning electron microscopy (W-SEM, JSM-6060, JEOL: accelerating voltage; 6 kV) and energy-dispersive X-ray spectroscopy (EDS, JED-2300, JEOL: accelerating voltage; 10 kV), respectively. The chemical analyses of the wear tracks of the 5-10-85 composite disks were performed using XPS after Ar+ ion etching for 30 s.
Figure 1 shows EDS images of a 5-10-85 composite surface before wear testing. Several pits on the surface of the 5-10-85 composite had formed during sintering and polishing process (Fig. 1(a)). In the EDS maps presented here, brighter regions have a higher concentration of the element being assessed, and both Si and Ti rich areas can be seen (Figs. 1(b) and 1(c)). During sintering of this material, the Si3N4 would be expected to react with titanium to produce titanium silicide (TiSix) such as Ti5Si3 and TiSi2, at high temperatures.30,36,37) Figure 2 presents the Si 2p peak profile of a 5-10-85 composite disk as obtained by XPS after Ar+ ion etching for 1000 s. The binding energy values of Si3N4, Ti5Si3 and TiSi2 are reported to be 101.7, 99.1 and 98.8 eV, respectively.38,39) XPS spectrum indicates that the Si in the 5-10-85 composite was primarily present as Ti5Si3 and TiSi2.

EDS images of the 5-10-85 composite surface: (a) a secondary electron image, (b) a Si Kα map and (c) a Ti Kα map. Brighter contrast indicates a higher concentration of the element.

The Si 2p peak profile obtained from the 5-10-85 composite using XPS.
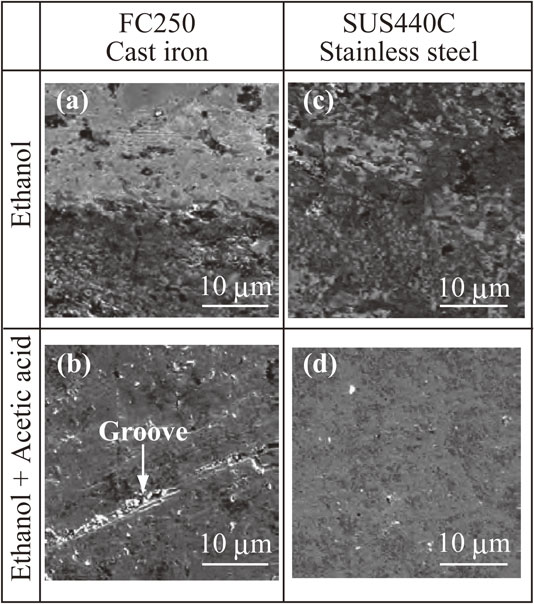
Figure 3 and Table 3 present the SEM images and the chemical composition and surface roughness values (Ra) of test specimens after immersion in ethanol with and without acetic acid, respectively. The surface of the FC250 cast iron disk after immersion in ethanol is smooth, and the dark grey areas in the image are attributed to graphite (Fig. 3(a)). In contrast, protruding white lamellar structures and hollows around the graphite flakes can be observed on the surface of the FC250 cast iron disk immersed in ethanol with added acetic acid (Fig. 3(e)). These white lamellae appear to be made of cementite (Fe3C) in perlite, and the protruding lamellar structure was likely formed by galvanic corrosion between the cementite and iron.11,40) The hollows appear to have resulted from galvanic corrosion between graphite and iron.11) Because of these protrusions and hollows, the Ra of the FC250 cast iron disk immersed in ethanol containing acetic acid was larger than that of the sample placed in the pure ethanol. The surface morphologies of the disks of the other materials were essentially the same following exposure to either pure ethanol or ethanol with acetic acid (Fig. 3). In addition, there were only minimal variations in the chemical compositions of the surfaces of the test specimens immersed in ethanol with and without acetic acid (Table 3).

SEM images of test specimens after immersion tests: the (a) FC250 cast iron disk, (b) SUS440C stainless steel disk, (c) titanium metal disk and (d) 5-10-85 composite disk after immersion in ethanol and the (e) FC250 cast iron disk, (f) SUS440C stainless steel disk, (g) titanium metal disk and (h) 5-10-85 composite disk after immersion in ethanol solution containing acetic acid.

Figures 4 and 5 show topographic images of the worn disk surfaces of ferrous materials and titanium-based materials, respectively. In each topographic image, brighter regions indicate higher positions and a cross sectional profile along the white horizontal line is shown.

Topographic images of the worn surfaces of disks of ferrous materials: FC250 cast iron disks worn in (a) ethanol and (b) ethanol solution containing acetic acid, SUS440C stainless steel disks worn in (c) ethanol and (d) ethanol solution containing acetic acid. Brighter contrast in each topographic image indicates a greater height.

Topographic images of the worn disk surfaces of titanium-based materials: titanium metal disks worn in (a) ethanol and (b) ethanol solution containing acetic acid, 5-10-85 composite disks worn in (c) ethanol and (d) ethanol solution containing acetic acid. Brighter contrast in each topographic image indicates a greater height.
The wear tracks of the ferrous materials worn in ethanol containing acetic acid were wider and deeper than those worn in ethanol (Fig. 4). The wear track of the FC250 cast iron disk worn in ethanol was groove shaped (Fig. 4(a)) and that worn in ethanol solution containing acetic acid was rough (Fig. 4(b)). Ridges can be seen in the middle of the wear track of the SUS440C stainless steel disk worn in ethanol (Fig. 4(c)) while grooves are apparent on the wear track of the SUS440C stainless steel disk worn in ethanol solution containing acetic acid (Fig. 4(d)).
Deep grooves are present in the wear tracks of the titanium metal disks worn in ethanol both with and without acetic acid (Figs. 5(a) and (b)). In the case of the 5-10-85 composite disks worn in ethanol with and without acetic acid, there are no evident differences in the surface configurations between worn and unworn surfaces (Figs. 5(c) and (d)).
Figure 6 summarizes the specific wear rates of the disks and pins, and provides error bars representing the standard deviation. In each case, the specific wear rate of the disk was calculated from the volume below the unworn surface. The wear of the disk of the ferrous materials in ethanol solution containing acetic acid were larger than those in ethanol. In particular, the specific wear rate of the SUS440C stainless steel disk in ethanol solution containing acetic acid was ca. 57 times as large as that in ethanol. The wear of titanium metal disk in ethanol and that in ethanol solution containing acetic acid were significantly large and about the same value. The wear volumes of the 5-10-85 composite disks in ethanol and in ethanol solution containing acetic acid were too small to be calculated using the cross-sectional area of the wear track.

Specific wear rates of disks and pins: (a) disk and (b) SUS440C stainless steel pin. The symbols “*” and “**” indicate that the wear could not be calculated.
Flat wear scars were observed on the sliding surfaces of SUS440C stainless steel pins slid against FC250 cast iron, SUS440C stainless steel and the 5-10-85 composite disks (data not shown). In contrast, titanium metal adhered on the hemispherical surface of the SUS440C stainless steel pins slid against titanium metal disks (data not shown). Therefore, the wear volume of pins slid against titanium metal disks could not be calculated. The wear of pins slid against FC250 cast iron, SUS440C stainless steel and the 5-10-85 composite disks in ethanol solution containing acetic acid was larger than that in ethanol.
3.4 Morphological and chemical analyses of worn surfaces 3.4.1 Ferrous materialsFigures 7 and 8 provide SEM and EDS images of the worn surfaces of FC250 cast iron and those of SUS440C stainless steel disks, respectively. Table 4 summarizes the chemical composition of the metallic area of FC250 cast iron disks and SUS440C stainless steel disks after the sliding test in ethanol with and without acetic acid. In this section, the data in Table 3 are treated as data of unworn surfaces.

SEM images and EDS maps of the surfaces of FC250 cast iron disks: (a) SEM image, (b) C Kα map and (c) O Kα map of the disk worn in ethanol and (d) SEM image, (e) C Kα map and (f) O Kα map of the disk worn in ethanol solution containing acetic acid. Brighter contrast in the EDS maps indicates a higher concentration of the element.

SEM images and EDS maps of the surfaces of SUS440C stainless steel disks: (a) SEM image, (b) O Kα map and (c) Cr Kα map of the disk worn in ethanol and (d) SEM image, (e) O Kα map and (f) Cr Kα map of the disk worn in ethanol solution containing acetic acid. Brighter contrast in the EDS maps indicates a higher concentration of the element.

The surface of the FC250 cast iron disk worn in ethanol exhibits plastic deformation (Fig. 7(a)) and a reduced concentration of C (Fig. 7(b)). These results indicate that plastically deformed iron covered the graphite on the surface of the FC250 cast iron disk worn in ethanol. An increased O concentration (Fig. 7(c), Tables 3 and 4) was also observed on the disk surface worn in ethanol. The sliding surface exhibits a high chemical activity because the mechanical energy due to friction generates frictional heat, dangling bond and fresh surface.41) The chemical reaction induced by friction is termed tribochemical reaction. Tribo-oxidation is a tribochemical reaction in which sliding materials were oxidized by oxidizing agents such as oxygen to form oxides on the sliding surface.42) It has been reported that iron oxides (Fe2O3 and Fe3O4) are produced by tribo-oxidation on the sliding surfaces of ferrous materials in the presence of oxygen and that iron can react with alcohols to form iron alkoxides that are only slightly soluble in alcohols.43–47) The iron oxide was obtained by the hydrolysis of the iron alkoxide.4,48) In our experiment, it appears that iron oxides were formed by the tribochemical reaction of iron with dissolved oxygen in the ethanol and/or with the ethanol itself.8)
The surface of the FC250 cast iron disk worn in ethanol solution containing acetic acid shows numerous hollows around the graphite flakes in addition to powdery debris (Fig. 7(d)). The hollows may have resulted from a galvanic corrosion between iron and graphite.8,11) The hard and brittle cementite lamellae were protruded by the galvanic corrosion.11,49) The powdery debris seemed to be the cementite fractured by sliding. The C and O concentrations of the worn and unworn surfaces of the FC250 cast iron disks in ethanol solution containing acetic acid were almost the same (Figs. 7(e) and 7(f)). On the worn FC250 cast iron disk surface, O was hardly detected (Table 4). Galvanic corrosion seemed to occur in preference to the reaction of iron with oxygen and ethanol on the sliding surface of the FC250 cast iron disk in ethanol solution containing acetic acid.
The surface of the SUS440C stainless steel disk worn in ethanol was plastically deformed (Fig. 8(a)) and oxidized (Fig. 8(b), Tables 3 and 4). The ridges in the middle of the wear track (Fig. 4(c)) appear to have been formed by the plastic deformation of the SUS440C stainless steel, while the oxidation of the sliding surface is ascribed to the reaction of iron with oxygen dissolved in ethanol and/or with the ethanol itself.4,43–48) The Cr concentrations on the worn and unworn surfaces were almost the same (Fig. 8(c)).
The surface of the SUS440C stainless steel disk worn in ethanol solution containing acetic acid was relatively smooth (Fig. 8(d)), and the O and Cr concentrations on the worn and unworn surfaces were almost the same (Figs. 8(e) and 8(f)). The addition of acetic acid to the ethanol resulted in increase of wear (Fig. 6(a)). These results suggest that dissolution of the sliding surface of the SUS440C stainless steel disk occurred in ethanol solution containing acetic acid.
Figure 9 shows SEM images of SUS440C stainless steel pins slid against ferrous materials. The SUS440C stainless steel pins slid against the FC250 cast iron disk and the SUS440C stainless steel disk in ethanol show oxygen-rich areas (dark area in Figs. 9(a) and 9(c)). Grooves can be observed on the worn surface of the pin slid against an FC250 cast iron disk in ethanol with acetic acid (Fig. 9(b)). The grooves were evidently formed by the abrasive action of the protruding and fractured cementite in the FC250 cast iron. The worn surface of the SUS440C stainless steel pin slid against the SUS440C stainless steel disk in the ethanol solution containing acetic acid was relatively smooth (Fig. 9(d)).
SEM images of SUS440C stainless steel pin slid against ferrous materials: SEM images of pins slid against FC250 cast iron disk in (a) ethanol and (b) ethanol solution containing acetic acid and SEM images of pins slid against SUS440C stainless steel disk in (c) ethanol and (d) ethanol solution containing acetic acid.
Figures 10 and 11 present SEM and EDS images of the worn surfaces of titanium metal and 5-10-85 composite disks. Table 5 summarizes the chemical compositions of these specimens after sliding test in ethanol both with and without acetic acid. Figure 12 provides SEM images of SUS440C stainless steel pins slid against the titanium-based materials.

SEM images and EDS maps of the surfaces of titanium metal disks: (a) SEM image, (b) O Kα map and (c) Fe Kα map of the disk worn in ethanol and (d) SEM image, (e) O Kα map and (f) Fe Kα map of the disk worn in ethanol solution containing acetic acid. Brighter contrast in the EDS maps indicates a higher concentration of the element.

SEM images and EDS maps of the surfaces of 5-10-85 composite disks: (a) SEM image, (b) O Kα map and (c) Fe Kα map of the disk worn in ethanol and (d) SEM image, (e) O Kα map and (f) Fe Kα map of the disk worn in ethanol solution containing acetic acid. Brighter contrast in the EDS maps indicates a higher concentration of the element.


SEM images of SUS440C stainless steel pins slid against titanium-based materials: pins slid against titanium metal disk in (a) ethanol and (b) ethanol solution containing acetic acid and pins slid against 5-10-85 composite disk in (c) ethanol and (d) ethanol solution containing acetic acid.
Many grooves and adhesive substances can be observed on the titanium metal disk surfaces worn in ethanol with and without acetic acid (Figs. 10(a) and 10(d)). These features are believed to have been produced by the cutting action of the mating pin and the adhesion of titanium metal to the mating pin, respectively. On the worn titanium metal disk surfaces, O and Fe were hardly detected (Figs. 10(b), 10(c), 10(e) and 10(f), Table 5). Titanium metal adhered to the surfaces of the SUS440C stainless steel pin slid against the titanium metal disk in ethanol both with and without acetic acid (Figs. 12(a) and 12(b)). These results demonstrate that wear of titanium metal was hardly influenced by the addition of acetic acid and that mechanical wear such as severe adhesion and cutting was the primary wear mechanism of the titanium metal disk in ethanol and in an ethanol solution containing acetic acid.
Both smooth and rough areas can be seen on the surfaces of 5-10-85 composite disks worn in ethanol with and without acetic acid (Figs. 11(a) and 11(d)). On the wear track, there were some areas whose concentrations of O and Fe were higher than those of unworn surface (Figs. 11(b), 11(c), 11(e) and 11(f)). EDS analysis revealed that the rough areas contained high concentration of O and small amounts of Fe (Table 5). The binding energies of Ti 2p3/2 peak of 458.6 ± 0.1 eV and O 1s peak of 530.4 ± 0.1 eV were detected in the XPS spectra of the wear tracks of the 5-10-85 composite disks worn in ethanol with and without acetic acid. These peaks were assigned to TiO2.50) The thickness of the titanium oxide layer on the wear track was less than the range distance of the electron beam of EDS.51) The elements both in the titanium oxide layer and in the substrate material were detected by EDS. Therefore, the atomic ratio of O/Ti in the rough area was smaller than that of TiO2, i.e., 2. On the surface of titanium-containing compounds slid in the presence of oxygen, titanium oxide (TiO2) was formed by tribo-oxidation.52) Titanium-containing compounds subjected to mechanical stimulation have also been found to react with alcohols to form titanium alkoxides.28,29,53,54) The titanium alkoxides subsequently hydrolyze and condense to form a titanium oxide (TiO2) gel with and without acetic acid.28,29,53–58) The increase in the O concentration in the rough areas of the worn surface of the 5-10-85 composite disk may have been caused by the formation of titanium oxide by the tribochemical reaction of the Ti component of the 5-10-85 composite with dissolved oxygen in ethanol and the ethanol itself. Films of titanium oxide and/or titanium oxide gel appear to have been present on the sliding surface of the 5-10-85 composite disks both in ethanol and in ethanol solution containing acetic acid. During sliding, the formation and removal of these films, i.e., tribochemical wear, seemed to occur.
Ti was hardly adhered on the SUS440C stainless steel pins slid against the 5-10-85 composite disks in ethanol both with and without acetic acid (Figs. 12(c) and (d)). O-rich substances, which might be iron oxide and/or chromium oxide, were observed on the surface of the SUS440C stainless steel pin slid against the 5-10-85 composite disk in ethanol (Fig. 12(c)). The surface of the SUS440C stainless steel pin slid against the 5-10-85 composite disk in ethanol solution containing acetic acid was smooth (Fig. 12(d)).
3.5 The effect of acetic acid on wear behaviorElectrolytes such as acetic acid are known to cause the electrochemical corrosion of metal materials in polar protic solvents.5) The films made of iron oxides (Fe2O3 and Fe3O4) on the cast iron, chromium oxide and iron oxide (Cr2O3 and Fe2O3) on the stainless steel and titanium oxide (TiO2) on the titanium-based materials acted as passivation layers that protected the metals from electrochemical corrosion such as anodic dissolution.13–17) During the sliding motion applied in our experiment, these passivation films appear to have been removed repeatedly. After the passivation films were removed, the highly reactive fresh surfaces were exposed.59) Then, the tribochemical reactions of bare metal with oxygen, ethanol and acetic acid took place on the fresh surface. Oxygen and ethanol formed metal oxide that contributed to repassivation. Acetic acid caused anodic dissolution of bare metal. Repassivation and anodic dissolution of the bare metal took place competitively on the sliding surface in ethanol solution containing acetic acid.
The addition of acetic acid resulted in increase of the wear of ferrous materials. On the sliding surface of the disk of ferrous materials in ethanol solution containing acetic acid, the anodic dissolution of iron seemed to dominant to repassivation. Therefore, wear caused by the anodic dissolution of iron took place on the sliding surface of the ferrous materials in ethanol solution containing acetic acid while wear caused by plastic deformation and oxidation of metals took place in ethanol.
On the other hand, the wear behavior of the titanium-based materials in ethanol was hardly affected by the addition of acetic acid. This is because the rate of growth of the repassivation film on titanium was higher than that on ferrous materials.60,61) Mechanical wear such as severe adhesion and cutting occurred regardless of the tribochemical reactions on the sliding surfaces of titanium metal disks both in ethanol and in ethanol solution containing acetic acid. The tribochemical reactions of titanium component with oxygen and ethanol generated titanium oxide on the 5-10-85 composite disks in ethanol both with and without acetic acid. The titanium oxide films seemed to show good passivity. Therefore, the 5-10-85 composite exhibited good corrosion and wear resistance in ethanol with acetic acid.
The effects of acetic acid in the lubricant on wear of the test specimens are summarized in Table 6.

Our experimental results can be summarized as follows.