2020 Volume 61 Issue 2 Pages 339-345
2020 Volume 61 Issue 2 Pages 339-345
The reaction of MgO with molten Al was investigated at 1000°C. When the MgO pellet was heated with Al in an alumina tube, MgAl2O4 spinel formed on both the MgO pellet and the inner surface of the alumina tube. Mg ions were reduced at the interface of the molten Al and MgO pellet, and spinel was formed, although the reaction demonstrated a positive change in the standard Gibbs free energy. The reduced Mg was dissolved in molten Al and reacted at the inner surface of the alumina tube to form the spinel. When Al was melted in the MgO tube, spinel formed at the inner surface of the tube. These reactions can be understood by considering an equilibrium reaction, 4MgO + 2Al $ \rightleftarrows $ MgAl2O4 + 3Mg, with activities of Mg and Al in the metal liquid. The formed spinel was black, which was due to the metal aluminum particles inside the formed spinel layer.
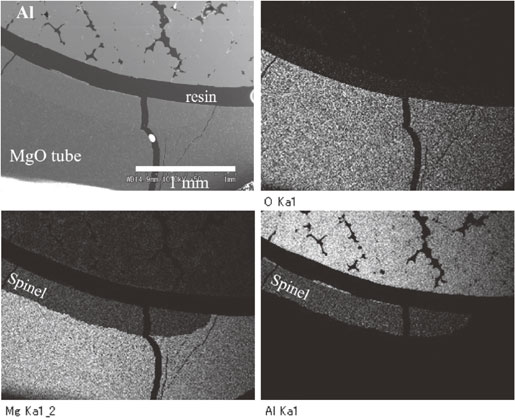
Fig. 7 SEM image and EDX element images of the MgO tube heated with Al at 1000°C for 25 h.
Magnesium oxide is used extensively as a refractory material. Although it has been mainly used in the iron and steel making industries, the interfacial reaction with other metals has also been investigated. Especially, the interface between the MgO and molten Al has been studied because of academic interest as well as its practical application in the Al alloy industry. In early investigations, it was reported that molten Al reacted with MgO to form MgAl2O4 spinel.1–3)
These investigations revealed that the formed spinel was black or blackish-grey, which was attributed to metallic Mg in MaAl2O4.2) According to a simple consideration of the reaction of MgO and Al at 1000°C, the formation of MgAl2O4, 4MgO + 2Al → 3Mg + MgAl2O4, i.e., the partial reduction of MgO by Al, is not favorable due to a positive standard Gibbs energy change of the reaction, ΔGr° > 0. To explain the result, the formation is attributed to a permeation of air through the porous crucible and/or a valorization of Mg.1) However, there is no explanation as to why MgO is reduced to form volatile species of metal Mg. Because MgO does not decompose into metal Mg and oxygen at 1000°C, the formation of Mg, i.e., reduction of MgO, must be explained thermodynamically. From a thermodynamic consideration of the existing liquid Al dissolving Mg, the three phases of MgO, MgAl2O4, and the liquid could be present at equilibrium at a higher temperature.3)
Other investigations mainly focused on the interface between MgO and molten Al. Experiments using a molten Al droplet on a MgO substrate were performed, and wettability and time dependence data, as well as the reaction at the interface were reported.4–10) Because MgAl2O4 spinel was formed at the interface, the crystallographic relationship between the formed spinel and MgO substrate was extensively investigated.7–10) These experiments were conducted using different orientations of a MgO single crystal. The spinel formation at the interface between Al2O3 and molten metal was also investigated. In this case, aluminum–magnesium alloy was mainly used and magnesium in the alloy react with Al2O3 to form spinel phase.11–13)
For practical applications of MgO refractory, further studies regarding the reaction of MgO with molten Al are necessary. Titanate ceramic refractories for the Al casting industry have been studied and the reaction of the ceramics with an Al alloy was reported.14,15) These results showed that the actual reactions sometimes cannot be explained by simple thermodynamic considerations.
In ordinary experiments using molten aluminum, an alumina crucible is often used, because the combination of aluminum and alumina is considered not to affect the reaction of the molten aluminum.16,17) Therefore, we studied the reaction of some oxide ceramics with molten aluminum in the alumina crucible.18) The results were, however, very interesting and the reaction of MgO with the molten aluminum was closely related to the reaction at the interface between the molten aluminum and the alumina crucible. Here, we present the results of our experiments of reaction of MgO and/or Al2O3 ceramics with molten Al and, discuss the role of the alumina and MgO crucibles and formation of the MgAl2O4 spinel phase.
For the fabrication of the MgO pellet, 99.9% pure Mg(OH)2 powder was used after calcination at 600°C for 2 h. The powder was CIPed at 100 MPa and fired at 1500°C for 2 h. A dense pellet (92.4% of the theoretical value) of approximately 7 mm in diameter and approximately 5.5 mm in thickness was obtained and used for the experiments. The pellet was placed at the bottom of the alumina tube, which was 99.9% pure with an inner diameter of 12 mm and an outer diameter of 15 mm. An 99% pure Al rod with a diameter of 10 mm was then put onto the MgO pellet in the tube. The used Al was approximately 5.5 g. After the melting test, the pellet located at the bottom of the tube was completely covered by Al.
In another experiment concerning the formation reaction of MgAl2O4, 5.5 g of the Al rod with diameter of 8 mm was put into an MgO tube and heated at 1000°C. The used MgO tube was 99% pure with an inner diameter of 10 mm and an outer diameter of 15 mm. The density of the tube was 93.3% of theoretical value, which was measured by the Archimedes’ method. The alumina or magnesia test tube was placed in a vertical ceramic tube furnace, and then heated. During the reaction test, 99.999% pure nitrogen gas at a rate of 100 mL/min was flowed. After the experiments, AlN was not detected by XRD in all cases.
After the test, the tube was cut, and the surface was examined by XRD (Ultima IV, Rigaku, Japan) using a CuKα radiation source, SEM-EDX (S-4300 and S-4800, Hitachi, Japan), and optical microscopy methods after polishing. A chemical analysis of the Al by ICP-AES (Ultima 2, Horiba Jobin Yvon, Japan) was conducted. In the chemical analysis, the Al (approximately 5 mg) was drilled from the polished surface, dissolved in aqueous HCl and then tested. Additionally, the Al was dissolved by hot aqueous NaOH, and the sample was also investigated.
Figure 1 shows a cross section of the MgO–Al reaction after 25 h, and Fig. 2 shows the XRD profile of black grains around MgO pellet after dissolution of the Al by hot aqueous NaOH. After the reaction test, the outer side of the MgO pellet became black with a thickness of approximately 0.9 mm. As shown in Fig. 1, cracks exist at the boundary of the black part and the central white part. After dissolving Al by a hot aqueous NaOH solution, the MgO pellet was easily broken into a central white block and outer black grains. An XRD measurement revealed that the outer black part consisted in MgAl2O4 with small amount of Al and periclase, as shown in Fig. 2. The MgAl2O4 spinel of the black grains was reported as a result of the reaction of the MgO crucible with molten alumina.1,2) The black color of the outer part of the MgO pellet is attributed to the existence of metal, as described in Ref. 2), and in present case, the metal was Al, as indicated by XRD. In the spinel layer, small white particles existed, as in Fig. 1, and they are Al and will be discussed later. The central white part is unreacted MgO. Cracks exist at the boundary between the spinel and MgO; these cracks are often observed during a solid-state reaction of Al2O3 and MgO to form spinel.19–21)
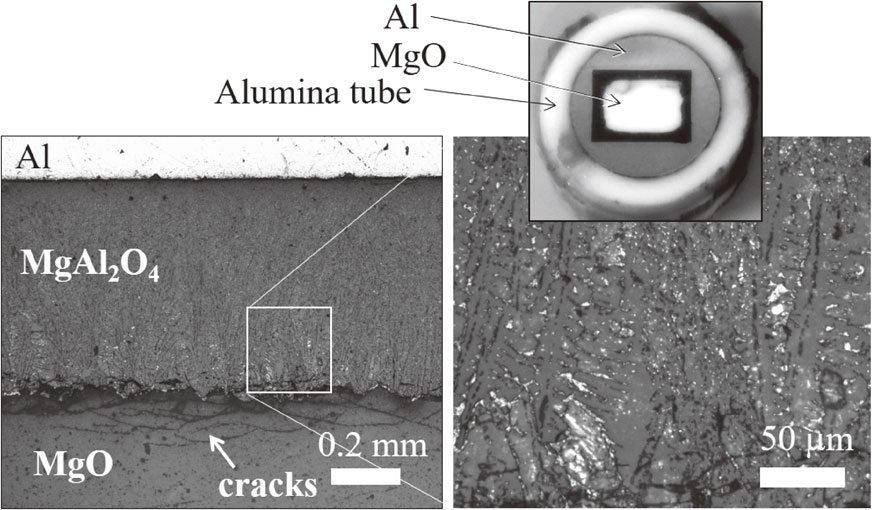
Cut and polished section of the MgO–Al interface tested in alumina tube.
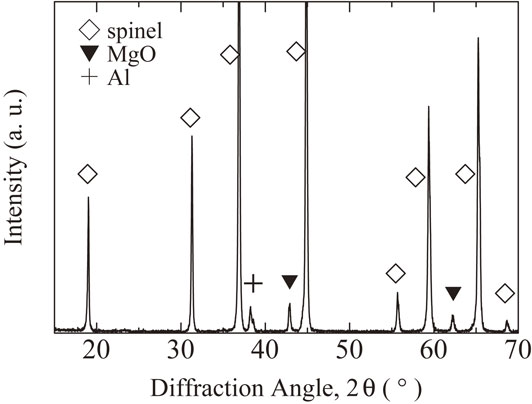
XRD profile of the broken black grains around the MgO pellet after the reaction test with molten Al in the alumina tube.
The appearance of aluminum solidified in the alumina tube after the experiment and the results of XRD measurement are shown in Figs. 3 and 4. The exposed surface to nitrogen stream of molten aluminum was oxidized by a small amount of oxygen in the nitrogen stream, and spinel and MgO were detected by XRD as shown in Fig. 4. In the cross section of the aluminum, only aluminum is detected, as shown in Fig. 4. The oxides cover the surface of molten aluminum and prevent further oxidation. The oxide layer is also presumed to prevent evaporation of the metallic species. Therefore, the interior of the molten aluminum could be regarded as a substantially closed system. The inner surface of the alumina tube used for the test turned black, which is the same as the surrounding part of the MgO pellet, as shown in Fig. 3. XRD experiments revealed that the inner part of the alumina tube consisted of MgAl2O4 spinel, corundum and a small amount of Al, as shown in Fig. 5. The thickness of the spinel layer in the polished cross section was observed to be 80–90 µm as shown in right photo of Fig. 3.
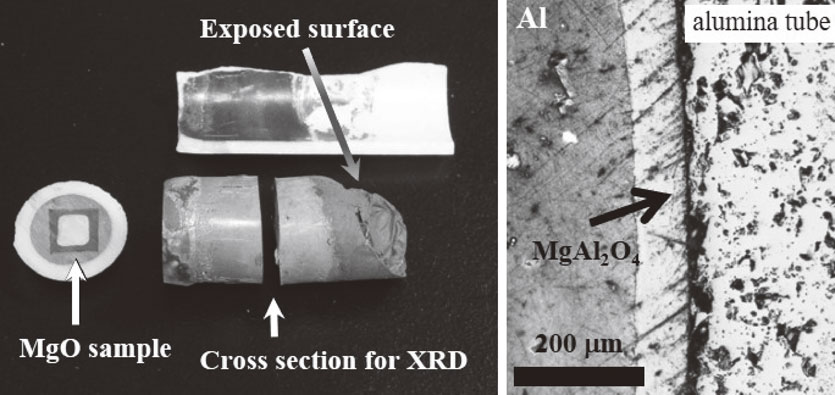
Solidified aluminum in alumina tube after the reaction test and cross-section of the interface between the alumina tube and aluminum.

XRD profiles of (a) cross section of Al and (b) surface of Al exposed to nitrogen stream after reaction for 25 h at 1000°C, ◇: spinel, ▼: MgO.
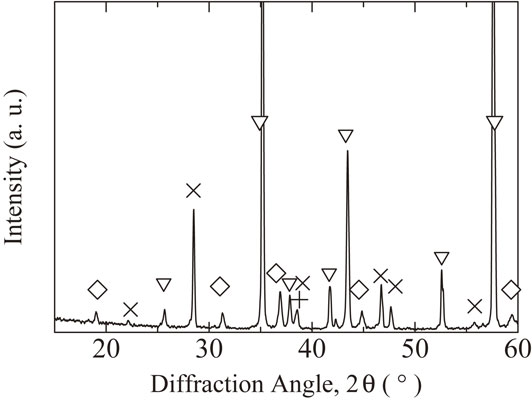
XRD profile of the inner surface of alumina tube after the reaction test for 25 h. ◇: spinel, ▽: corundum, +: aluminium, and ×: adhesive used for fixing the sample.
The formation of spinel layer at the inner surface of alumina tube suggested that Mg is supplied from the MgO pellet through the molten aluminum. The EDX analysis showed that the aluminum contained approximately 3 at% Mg. The two ICP-AES analyses also showed that Al contained 2.2 and 2.1 at% of Mg after the reaction test. In a blank test, in which only Al was heated in the alumina tube for 25 h at 1000°C, Mg was not detected by ICP-AES. To explain the dissolution of Mg in Al and the formation of the spinel, the following reaction of MgO with Al to form Mg and Al2MgO4 is considered.
| \begin{equation} \text{4MgO} + \text{2Al} \to \text{MgAl$_{2}$O$_{4}$} + \text{3Mg}. \end{equation} | (1) |
The change in the standard Gibbs energy of this reaction at 1000°C is calculated as a positive value of ΔGr° = 66.7 kJ, and all the thermodynamic data hereafter were from a textbook of Kubaschewski.22) We have already reported that reaction (1) can be realized at equilibrium by considering the Al–Mg liquid.18) However, there was an additional reaction of spinel formation at the inner surface of the alumina tube. Regarding this spinel formation, the reaction below occurred.
| \begin{equation} \text{4Al$_{2}$O$_{3}$} + \text{3Mg} \to \text{3MgAl$_{2}$O$_{4}$} + \text{2Al}. \end{equation} | (2) |
The change in the standard Gibbs energy of this reaction at 1000°C is calculated as ΔGr° = −258.3 kJ. If the two reactions (1) and (2) occur in a system, then a very simple reaction of the formation of the MgAl2O4 spinel from Al2O3 and MgO is obtained, and ΔGr° = −47.9 kJ/mol. This reaction is thermodynamically favorable.
In the system of MgO immersed in molten Al in an alumina tube, the usual reaction of spinel formation from MgO and Al2O3 occurs, but the transport mechanism is somewhat complex; Mg2+, initially from MgO, is reduced at the interface of molten Al/MgAl2O4 of the MgO side by Al as reaction (1), and then Mg is dissolved into Al. At the interface of molten Al/MgAl2O4 of the alumina tube side, Mg is oxidized to Mg2+ ion and reacts according to reaction (2) to form spinel. The same reduction of MgO to metal Mg was also reported in the reaction of molten steel deoxidized by Al with MgO to form spinel inclusion.23)
The solid-state reaction of MgO with Al2O3 to form MgAl2O4 spinel was extensively studied, and the thickness of the formed spinel on the MgO side and the Al2O3 side was 1:3–5, depending on the composition of the formed spinel.19,24,25) If the formed spinel had a composition of MgAl2O4, the ratio was 1:3, as the ratio of reactions (1) and (2). The content ratio of the formed spinel on the MgO pellet and alumina tube was roughly estimated. In the present case, the thickness of the spinel formed around the MgO pellet was approximately 0.9 mm, and the thickness of the spinel on the inner surface of the alumina tube was approximately 80 µm. The inner area of the alumina tube was estimated by the volumes of molten Al and the MgO pellet. The resultant volumes of the spinel on the MgO pellet surface and alumina tube are 160 mm3 and 200 mm3, respectively, which was already described in Ref. 18). The larger amount of spinel formed on the inner surface on the alumina tube was a very rough estimation but was a comparable amount. This finding suggested that the formation reactions of spinel were reactions of (1) and (2). Although the thickness of the spinel layer formed on the inner surface of the alumina tube was thin, the amount of spinel was larger owing to the wider area of the inner surface of the tube.
3.2 Melting experiment of aluminum in the MgO tubeThe abovementioned reaction was thermodynamically favored reaction due to the formation of the spinel on the inner surface of the alumina tube. Thus, an experiment without the alumina tube was examined. Aluminum was placed in the MgO tube and heated at 1000°C under the nitrogen flowing condition. After the test, white powder deposited on the part of the surface of molten aluminum and this powder was periclase, which was detected by XRD measurement. The formation and evaporation of metal Mg, and oxidation by oxygen in the nitrogen stream caused the formation of periclase powder. But the amount of the white powder did not increase with time and the most surface of the aluminum was covered with spinel as the same as the test using the alumina tube and sintered MgO sample.
A typical result of the sample heated for 25 h is shown in Fig. 6. After the experiment, the inner part of the tube became black, which is the same as the MgO pellet heated with aluminum in alumina tube. An XRD experiment revealed that this part consisted of MgAl2O4 spinel, periclase and Al, which was shown in Fig. 6. The periclase is due to the unreacted MgO tube.
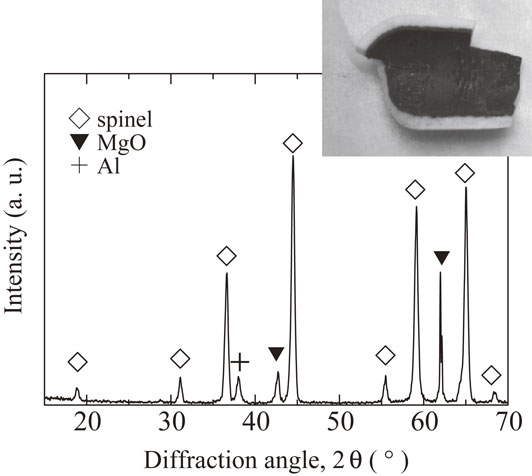
XRD profile of the inner surface of MgO tube after the reaction test for 25 h, and the MgO tubes after the reaction for 25 h (inserted).
The tube was cut and polished, and then was observed by SEM and EDX, as shown in Fig. 7. EDX images showed the layer formation of the spinel phase on the inner surface of the MgO tube. The thickness of the spinel layer was almost the same as the formed part. However, the layer was not always formed throughout the inner surface. Large cracks are observed in Fig. 7, and the cracking hardly affected the formation of MgAl2O4. Cracking occurred in the sample of a shorter soaking time.
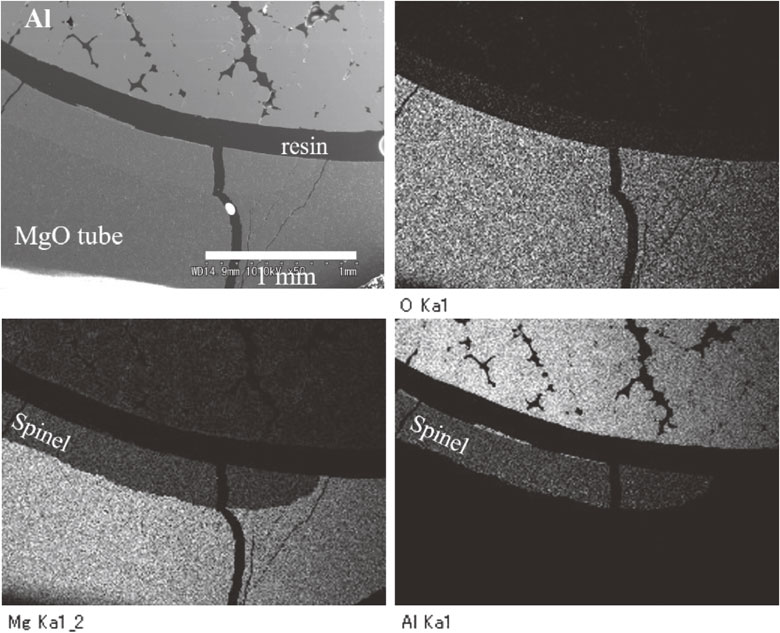
SEM image and EDX element images of the MgO tube heated with Al at 1000°C for 25 h.
Figure 8 showed SEM and EDX images of the interface between the remaining MgO and formed spinel in the MgO tube sample being heated for 12 h. The EDX element images of Al and O suggest the existence of metal Al in the reacted spinel layer. The existence of metal particles was also suggested by the XRD results in Fig. 6. An optical reflection microscope clearly exhibited the existence of metal aluminum and the micrograph was shown in Fig. 9. In the micrograph, white particles existed in the spinel layer and these are metal aluminum. The aluminum particles in the spinel layer were small, about 1–2 µm in size, and others were irregular shape. Some spherical particles with about 5 µm diameter should be aluminum filled in pores of the used MgO tube. Large aluminum particles existed near the boundary of the MgO and formed spinel. Because the used MgO tube was dense, about 93.3% of theoretical density, the aluminum hardly enters inside the tube through open pores. The grain size of MgO of the used tube was about 30 µm and the aluminum showed smaller distribution. This suggested that aluminum did not penetrate through the grain boundaries. The reaction front inside the MgO tube and MgO sintered body confirmed that the reaction is free from the vaporization of Mg from the liquid aluminum surface. The white particles shown in Fig. 1 were also the aluminum particles, as shown in Figs. 8 and 9.
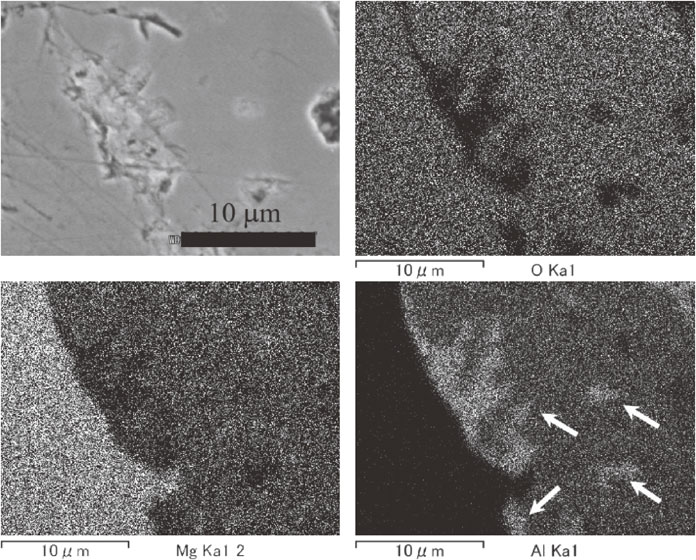
SEM image and EDX element images of the interface between MgO and formed spinel, heated 1000°C for 12 h. Arrows show some metal Al particles.

Optical reflection microscope image of the interface between unreacted MgO and formed spinel, heated 1000°C for 12 h. The sample surface was polished and etched with Ar+ ion.
The cracks are likely caused by the change in volume during the reaction test and/or cooling period to room temperature. The volume change accompanied with the formation of spinel from MgO and Al, shown as reaction (1), is estimated. Because Al and Mg forms liquid at the test temperature, the volume change will be accompanied by the change of solids from 4MgO to MgAl2O4. This change is calculated to be −13.0 vol%, assuming a lattice constant at RT of 4.211 and 8.080 Å and linear thermal expansion from RT to 1000°C of 1.36 and 0.86% for MgO and MgAl2O4 spinel, respectively.26,27)
If the cracks are formed by this volume change, then it caused the crack opening at the inside of the tube but in many cases the cracks opened outside of the tube. Additionally, there was no leakage of Al and the crack did not influence the formation of the spinel, as shown in Fig. 7. Therefore, this volume change is not the reason for the cracking. Another reason for the cracking is the difference in the thermal contraction of the MgO and the spinel. MgO has very large thermal expansion coefficient, and the large contraction of the MgO tube during cooling from the test temperature caused the cracking.
It is interesting that the large volume contraction accompanied by the formation of the spinel from MgO hardly caused the cracking. As already described in many papers, the interface between the MgAl2O4 spinel and MgO easily separates after the solid-state reaction of Al2O3 with MgO, and the spinel seldom has a crystallographic relationship with MgO. The spinel formed stress-freely on the inner surface of the MgO tube and the spinel layer was porous, −13% in volume change, to allow a penetration of Al into the spinel layer as shown in Figs. 1, 8 and 9, and Ref. 8).
3.3 Discussion regarding the formation of the spinel on the MgO tubeThe Mg content in Al was measured using ICP-AES from the polished surface of a sample heated for 25 h. The two measurements resulted in Mg contents of 7.0 and 6.5 at%. The Mg content measured by EDX is plotted against soaking time t at 1000°C, and the result is shown in Fig. 10. Four measurement points were used in the EDX measurements at 200 µm intervals. The semi-quantitative analysis by EDX of the sample also showed a larger amount of Mg than in the former experiment using a MgO pellet in the alumina tube, about 2 at%. The amount of Mg in molten Al was approximately three times larger than the sample in the alumina tube, mentioned in the previous section. This behavior indicates that the dissolved Mg in Al (according to the reaction (1)) is consumed to form the spinel on inner surface of the alumina tube by reaction (2). The thickness of the spinel layer d was also measured by 4 points at a 90° rotation, and the results are shown in Fig. 11 against soaking time t. The thickness of the spinel layer increased with soaking time but seemed to saturate at a longer duration of 9 to 10 h.

Mg concentration measured by EDX (○) as a function of heating time at 1000°C and by ICP-AES (■ without error bar).

Thickness of the spinel layer versus heating time at 1000°C.
The experiment clearly demonstrated that the spinel formation reaction of MgO with Al proceeded, even if there was no reaction of the spinel formation reaction of Al2O3 and Mg, namely reaction (2). To explain this, we simply consider the law of mass action. Because Al and Mg are melted, we have the following eqs. (3) and (4) for an equilibrium reaction (1′).
| \begin{equation} \text{4MgO} + \text{2Al} \rightleftarrows \text{MgAl$_{2}$O$_{4}$} + \text{3Mg}. \end{equation} | (1′) |
| \begin{equation} K = \frac{[\text{MgAl$_{2}$O$_{4}$}]a_{\text{Mg}}{}^{3}}{[\text{MgO}]^{4} a_{\text{Al}}{}^{2}} \end{equation} | (3) |
| \begin{equation} \Delta G = \Delta G_{\text{r}}^{\circ} + RT\ln K \end{equation} | (4) |
Hallstedt thermodynamically calculated a phase diagram of MgO–Al2O3.30) The resultant phase diagram was consistent with the experimental data. The diagram showed a sharp increase in the solid solubility of Al2O3 into spinel phase above 1600 K and a very narrow solubility range of spinel below 1300 K. The activity of spinel having near stoichiometric composition was almost unity. Therefore, we here regard the activity of spinel as unity.
When the activity of the solids is unity and ΔG = 0 at equilibrium, the equations below are derived.
| \begin{equation} RT\ln K = -\Delta G_{\text{r}}^{\circ} = - 66.7\,\text{kJ} \end{equation} | (5) |
| \begin{equation} K = \frac{a_{\text{Mg}}{}^{3}}{a_{\text{Al}}{}^{2}} = 0.00183 \end{equation} | (6) |
If the activity of the component is the same as its molar fraction, aAl = 0.887 and aMg = 1 − aAl = 0.113, then the Mg concentration results are approximately 11 at%. Of course, because the Al–Mg solution is not an ideal solution, the concentration is not always 11 at%. However, the calculated concentration is not very different from the measured values, i.e., 6.5 to 7.0 at% by ICP-AES. At the equilibrium among the MgO, MgAl2O4, and liquid, the amount of the phases has no meaning, and only the molar fraction of the liquid is important. But as shown in the reaction (1′), an increasing concentration of Mg in the liquid is accompanied with the increasing amount of MgAl2O4.
The saturating tendency of the Mg content in the liquid, shown in Fig. 10, suggested the system reached near the equilibrium condition. The spinel formation reaction involves a change in Mg content in the liquid, therefore the formation reaction of spinel substantially stopped as shown in the saturating spinel thickness in Fig. 11. Although the evaporation of Mg metal from the liquid will shift the reaction (1′) rightward to increase the amount of spinel, the surface of the liquid is covered with the oxide layer of spinel and the evaporation is limited. The difference in the time of saturation between the Mg concentration, about 7 h of the reaction, and the thickness of spinel layer, about 9 to 10 h, would suggest the effect of the evaporation on the reaction (1′). After the saturation of Mg in the liquid in 7 h, the vaporization of Mg still proceeded to 10 h, and the amount of spinel increased as the equilibrium shifted to the right. In the time of 10 h, the liquid was almost covered with the oxide, and the vaporization became little and the amount of the spinel saturated.
During the early stage of the reaction, Mg was not detected in Al, and there was no spinel layer. This effect was caused by the lack in the wettability of molten Al within the MgO tube during the early period of the reaction test. After 4 h, the Mg content increased, and the spinel layer started to form.
It is interesting that the maximum thickness of the spinel layer reached 250 µm after 4 h, and a very thick spinel layer formed instantaneously. Usually, the thickness of the spinel layer by the solid-state reaction of Al2O3 with MgO was reported as approximately several hundred micrometers at 1500–1600°C for several tens of hours of reaction time18,19,31). The present spinel layer was obviously thick compared with these values. This behavior is attributed to the volume contraction resulting from the formation reaction of the spinel from MgO, as mentioned above. The large contraction in volume resulted in a porous spinel, and the metal liquid of Al–Mg was immersed into the pore. This metal liquid enhanced the formation reaction (1′), and a thick spinel layer resulted. The penetrated metal particles in the spinel were detected by XRD, as shown in Fig. 6, and observed by the microscopic observations, as shown in Figs. 1, 8, and 9.
MgO reacts with Al to form a MgAl2O4 spinel at 1000°C. Although the simple reaction of MgO with Al to form MgAl2O4 and metal Mg has a positive standard Gibbs energy change, an equilibrium condition, 4MgO + 2Al $ \rightleftarrows $ MgAl2O4 + 3Mg, can be realized with aAl = 0.887 and aMg = 0.113 at 1000°C, where aAl and aMg are the activities of Al and Mg in the liquid, respectively.
When a MgO pellet is heated with Al in an alumina tube at 1000°C, spinel layer forms on both the surface of the MgO pellet and the inner surface of the alumina tube. Mg2+, initially from MgO, are reduced at the interface of the molten Al and MgO to form the spinel, and then Mg is dissolved into the molten Al. At the interface of the molten Al and alumina tube, Mg is oxidized to Mg2+ and reacts with alumina to form the spinel.
The formed spinel layer is black because of the metal Al particles in the layer. The metal particles can penetrate the spinel layer, because the layer is porous due to a volume contraction accompanied by the spinel formation reaction. The porous spinel and penetration of metal Al cause very high formation rate of spinel comparing a solid MgO-alumina reaction.