2020 Volume 61 Issue 2 Pages 368-374
2020 Volume 61 Issue 2 Pages 368-374
The aim of this study was to clarify the structural evolution of porous aluminum during brazing with an Al–Si-based alloy brazing sheet. High-quality metallurgical bonds between porous aluminum and solid aluminum sheets are required for effective thermal management applications. The authors have proposed a new brazing method using an Al–Si-based alloy brazing sheet that can control the liquid metal for the bonding in smaller amounts. Although the metallurgical bond was realized without filling the pores, the new brazing method influenced the cell structure because of liquid migration. The liquid migration and cell structure evolution in sintered dense aluminum and porous aluminum were experimentally investigated as a function of brazing time. The experimental results indicated that the liquid migration distance in both the dense and porous aluminum obeyed a parabolic law. The velocity of liquid migration in the porous aluminum was higher than that in the sintered dense aluminum, attributed to the high cell wall surface area in the porous aluminum. During liquid migration, the liquid filled former powder boundaries before and after grain coarsening occurred. Therefore, grain coarsening was attributed to liquid film migration, which directly affected evolution of the cell structure. The grain diameter was linearly related to the porosity, where extrapolation of this relationship showed that the grain diameter was equal to the diameter of the raw aluminum powder when the porosity was equal to the initial porosity.
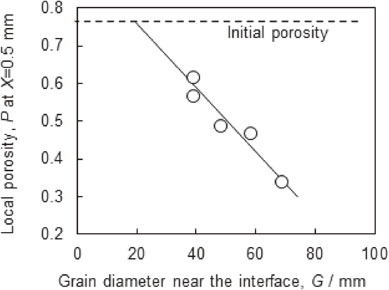
Local porosity (P) at X = 0.5 mm for various times as a function of the grain diameter near the interface (G).
High-quality metallurgical bonds between porous aluminum and solid aluminum sheet are required for effective thermal management applications, such as motors, batteries, and manufacturing equipment which generate heat. The interface of the metallurgical bond more efficiently transfers heat compared with adhesive bonds. The use of aluminum with an open porous cell structure can be used to enhance the heat transfer rate of heat storage materials, such as phase-change materials (PCMs).1–3) A previous study4) demonstrated metallurgical bond between porous aluminum and solid aluminum sheet by soldering, which is a similar method to brazing. During soldering, the liquidus temperature of the filler materials is under 723 K, while in the case of brazing, the corresponding liquidus temperature is higher than 723 K.5) In these bonding methods, the filler materials melt and flow into the bonding interfaces. However, when open-cell porous aluminum is bonded by brazing or soldering, the liquid metal tends to fill the pores under the effect of capillary forces. This results in a loss of porosity and original cell structure when large amounts of liquid metal are present. Such changes degrade the heat-storage properties, such as the heat transfer rate. Apart from this study, there seems to be little research on the metallurgical bond between porous aluminum and solid aluminum sheets.
We previously proposed a new brazing method6) using an Al–Si-based alloy brazing sheet that limits the liquid metal volume during brazing to prevent pore filling. The new brazing method does not require a filler material. Liquid that is partially formed in the Al–Si-based alloy sheet during brazing behaves as the bonding phase. The amount of liquid formed in the Al–Si-based alloy sheet is controlled by the chemical composition of the Al–Si-based alloy sheet and the brazing temperature. Although metallurgical bond was achieved without filling the pores, the cell structure, especially near the interface, is changed during brazing with this method,7) which could degrade the heat-storage performance.
In the new brazing method, some of the Al–Si-based alloy brazing sheet melts at above the solidus temperatures and the liquid migrates into the interface between the porous aluminum and the brazing sheet. The liquid fills the clearance and forms a metallurgical bond at the interface. Unfortunately, the liquid can further migrate into the porous aluminum, reacting with the cell walls and changing the cell structure. We previously suggested7) that the mechanism of liquid migration in the porous aluminum during the brazing process followed three steps: 1) liquid spreads on the surface of the cell walls; 2) the liquid penetrates cell walls via former powder boundaries; and 3) grain coarsening occurs via liquid film migration (LFM)8,9) in the cell walls. However, the manner in which the liquid migration affects the cell structure was not clear because time variation was not investigated. To prevent cell structure evolution, revealing the mechanism of liquid migration and clarifying the root cause of the cell structure evolution were essential.
This study aimed to clarify changes in the porous aluminum cell structure during brazing with Al–Si-based alloy brazing sheet. The liquid migration in the sintered dense aluminum and porous aluminum was experimentally investigated as a function of brazing time. The sintered dense aluminum was used as a simple model of the cell walls in the porous aluminum. The time variation of the liquid migration and cell structure evolution during the brazing demonstrated the detailed mechanism of both phenomena. These results indicated a dominant factor of the cell structure evolution and could help in developing measures to prevent cell structure evolution.
Aluminum powder (99% purity; average grain size ∼20 µm; Kojundo Chemical Lab. Co., Ltd.) was used to produce the sintered dense aluminum, while the same aluminum powder mixed with NaCl powder (95% purity; average grain size 330–430 µm; Naikai Salt Industries) were used to fabricate the porous aluminum. Table 1 shows the process parameters used to produce the sintered dense aluminum and porous aluminum materials. The porous aluminum samples were processed using a sintering–dissolution process that included mixing, compacting, sintering, cutting, and leaching.10) First, the raw materials were measured (Al:NaCl = 30:70 at volume percentage in the case of porous aluminum, aluminum is 100% in the case of sintered dense aluminum) and mixed in a ceramic bowl under a dry atmosphere. Then, the mixed powder was filled into the columnar carbon mold which had an inner diameter of 10 mm. After that, cold compaction was conducted using a hydraulic press with a pressure of 30 MPa in the axial direction. The green compacts were sintered using spark plasma sintering equipment (PLASMAN, S-S-ALLOY, Japan) with a pressure of 30 MPa in the axial direction under a vacuum atmosphere of less than 100 Pa. To remove surface contamination, the sintered sample was cut with a turning machine. Finally, leaching was carried out in running water to remove the NaCl powder and achieve a porous sample. The leaching step was not required for the sintered dense aluminum, but all other processes conditions were the same as those used for the porous aluminum. The diameter and height of all prepared samples were 8 mm and 4.5 mm, respectively. The target porosity of the porous aluminum was 70%.
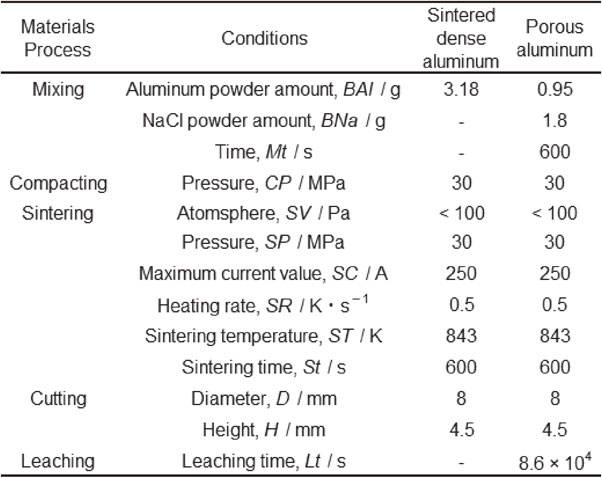
The chemical composition of the 1-mm-thick Al–2.5Si brazing sheets used in this study is shown in Table 2. The brazing sheets were manufactured by rolling, and then tempered under H1211) conditions. Additionally, commercial 1-mm-thick A3003 aluminum sheets were prepared as a jig material. Both types of sheets were fabricated in our laboratory.
A schematic diagram of the brazing test pieces used here is shown in Fig. 1. Square samples with dimensions of 30 mm × 30 mm were used for both the Al–2.5Si and A3003 aluminum sheets. First, the Al–2.5Si sheet was coated with a liquid flux solution containing 3-mass%-fluoride-series-flux, and then baked to evaporate the liquid at 473 K for 3600 s in an electric furnace. After that, the test pieces were assembled and brazed under a controlled atmosphere. In brazing process, the oxygen concentration was less than 1.0 × 10–3%, the dew point was less than 213 K, the brazing temperature was 863 K and the brazing times were 20, 60, 180, 600 and 1800 s. The effect of brazing time (20–1800 s) was investigated. Considering the equilibrium phase diagram12) and lever rule, the liquid fraction of the Al–2.5Si brazing sheet at 863 K is 9.8%.
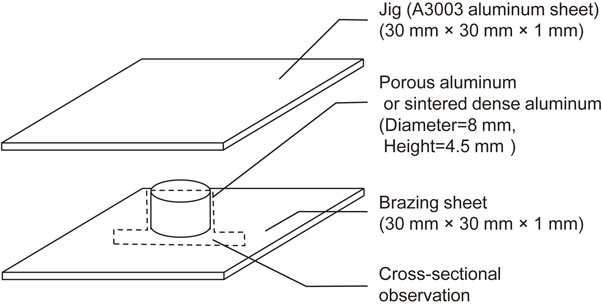
Schematic diagram of the brazing test piece (either porous aluminum or sintered dense aluminum) prepared in this study.
We observed the microstructure of the test pieces using optical microscopy (OM). In order to observe the cross-sectional microstructures, the test pieces were cross-sectioned along the dashed line shown in Fig. 1. After polishing, anodic oxidation using Barker’s solution (HBF4:H2O = 1:30) was conducted to visualize the grain structure. Then, the structures were observed using OM. In addition, elemental maps were produced using an electron probe microanalysis (EPMA).
Microscopy images are shown in Fig. 2, which define the liquid migration distance (L) and grain diameter near the interface (G) used here. The bonding interface was determined by connecting the sheet surfaces of the unbonded portions with straight lines. Region 1 is the area affected by the liquid, which appears darker in the image compared to Region 2 (without migrated liquid). The distances from the bonding interface (dashed white line) to the boundary between regions 1 and 2 (solid white line) were defined as L. As shown in Fig. 2, there was also liquid migration from the sidewall. In order to avoid the effect of sidewall migration, L was measured at the center of the sintered dense aluminum and porous aluminum samples.
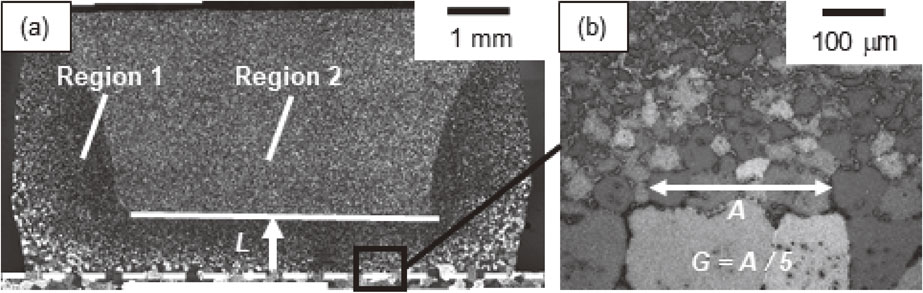
Representative microscopy images showing the measurement method for (a) liquid migration distance (L) and (b) grain diameter near the interface (G).
The G value was calculated by averaging the width (A) of five randomly selected grains (as indicated by the white arrow in Fig. 2(b)). The G value was measured using the grains closest to the interface. Three sets of G values for each brazing time were measured and the average values were calculated.
The local porosity of porous aluminum (P) was measured from low-magnification OM images at various distances from the bonding interface (X). The X values ranged from 0.5 mm to 3.5 mm. The P values were measured over an area of 4.0 mm (width) × 0.8 mm (height) at each X using Image J image processing software.13) The P values were calculated by dividing each pore area by the measurement area (3.2 mm2).
The microstructural changes in the sintered dense aluminum after various brazing times are shown in Fig. 3. The initial microstructure (Fig. 3(a)) showed a uniform microstructure and fine grains, while after brazing (Fig. 3(b)–(f)), a darker region (corresponding to the migration of the liquid from the brazing sheet) was observed near the bonding interface and sidewall that grew inward with increasing brazing time. The white arrows in Fig. 3(b)–(d) indicate the liquid migration distance (L). In Fig. 3(e) and (f), no arrows were included as drawn in the pictures because the L values exceeded the observation range. The grains near the interface and sidewall coarsened with increasing brazing time.

Microstructure change of the sintered dense aluminum (a) before brazing, and after (b) 20 s, (c) 60 s, (d) 180 s, (e) 600 s, and (f) 1800 s of brazing.
Elemental maps were produced to verify the elemental composition of the darker region observed during microscopy. Figure 4 shows (a) a backscattered electron image (BEI), and elemental maps of (b) Al and (c) Si obtained using EPMA in the sintered dense aluminum after 180 s of brazing. We chose to analyze the Si distribution as this element was only present in the Al–2.5Si brazing sheet, and is a direct indicator of liquid migration during brazing. As shown in Fig. 4(c), Si was distributed near the interface and sidewall of the sintered dense aluminum and corresponded well with the dark region shown in Fig. 3(d). Hence, the dark regions in the OM images were due to a liquid from the brazing sheet.
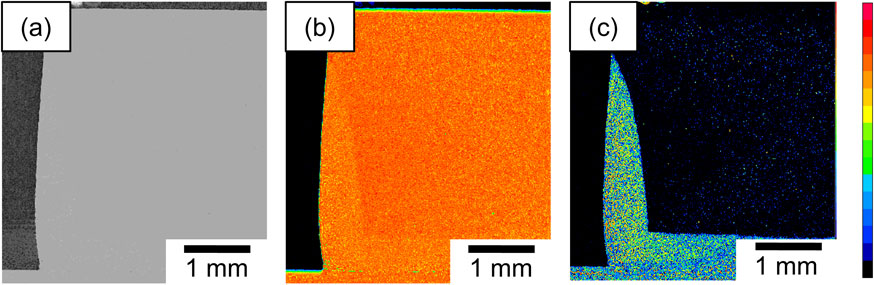
(a) EPMA backscattered electron image and elemental maps of (b) aluminum and (c) silicon in the sintered dense aluminum after 180 s of brazing.
The microstructural changes in the porous aluminum after various brazing times are shown in Fig. 5. As shown in Fig. 5(a), the microstructure before brazing was uniform, with fine grains in the cell walls, and pore diameters <500 µm. Figures 5(b)–(f) show that the number of pores decreased and the grain diameter of the cell walls increased with increasing brazing time.
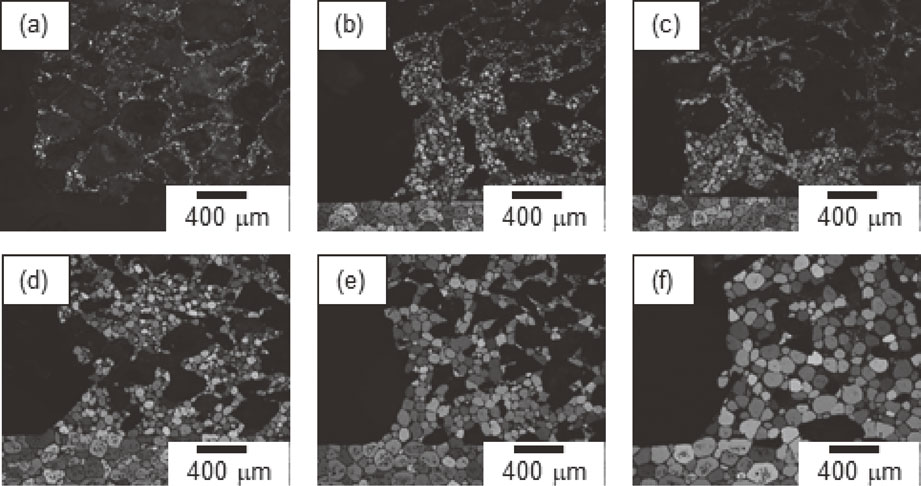
Microstructure of the porous aluminum (a) before brazing, and after (b) 20 s, (c) 60 s, (d) 180 s, (e) 600 s, and (f) 1800 s of brazing.
Figure 6 shows (a) BEI and elemental maps of (b) Al and (c) Si determined using EPMA for the porous aluminum after 180 s of brazing. As shown in Fig. 6(c), Si was widely distributed in the cell walls. The Si concentration near the interface was equivalent to that in the brazing sheet. The Si concentration away from the interface was higher than that in the original brazing sheet. These results indicate that the liquid supplied by the brazing sheet preferentially migrated to the cell walls.

(a) EPMA backscattered electron image and elemental maps of (b) aluminum and (c) silicon in the porous aluminum after 180 s of brazing.
The liquid migration velocity in the sintered dense aluminum and porous aluminum was evaluated by measuring L at various brazing times. Figure 7 shows L in both the solid and porous aluminum as a function of the square root of brazing time (t). The dashed line in Fig. 7 shows the initial height of the samples. The lines of best fit shown with the data points indicate that L was linearly proportional to $\sqrt{t} $. The slope for the porous aluminum samples was approximately five times higher than that of the sintered dense aluminum, indicating a higher liquid migration velocity in the porous sample.

Liquid migration distance (L) in the sintered dense aluminum and porous aluminum as a function of the square root of the brazing time (t).
The grain coarsening behavior in the sintered dense and porous aluminum samples were compared with respect to the grain diameter near the interface (G) after various brazing times. Figure 8 shows data for G, along with lines of best fit, as a function of $\sqrt{t} $ for both sample types. As for G, a linear relationship between G and $\sqrt{t} $ was observed for both the dense and porous aluminum. The G values and the slope were similar for both sample types, indicating that grain coarsening mechanism was similar in both.

The grain diameter near the interface (G) of the cell walls in the sintered dense aluminum and porous aluminum as a function of square root of the brazing time (t).
Changes to the cell structure of the porous aluminum after brazing were evaluated by measuring the local porosity (P). Figure 9 shows P as a function of various brazing time and distance from the interface (X). The value of P before brazing was constant at various X values, while P for a specific brazing time decreased with decreasing X. The porous aluminum was brazed to both the brazing sheet and the jig after brazing for 1800 s because the brazing time was too long and the liquid formed a metallurgical bond with the jig. Therefore, P at X = 3.5 mm was not measured due to the influence of liquid accumulating near the jig. In addition, P decreased with increasing brazing time. Based on these results, we can say that the optimal brazing time is 20 s in this study. In addition, it is possible to prevent the reduction of P at a shorter brazing time. However, if the brazing time is too short, metallurgical bonds may not be formed. Further research is required to clarify this point.
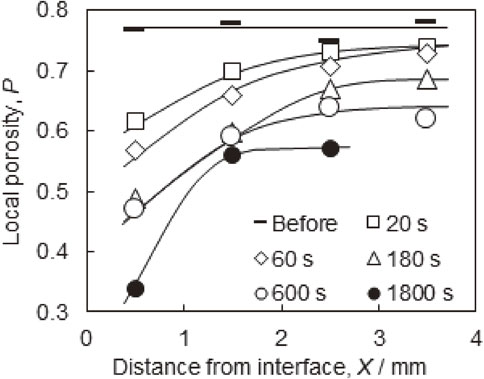
Local porosity (P) of the porous aluminum after various brazing times as a function of the distance from interface (X).
During liquid spreading, the cell wall surface area significantly influences L. As shown in Fig. 7, both lines of best fit were proportional to $\sqrt{t} $, indicating that liquid migration occurred in both samples via a constant driving force. We assume that liquid migration was due to capillary effects, driven by surface tension.14) The Lucas–Washburn equation describes the capillary phenomenon by a simple column model, where L is proportional to $\sqrt{t} $.15–17) As the only significant difference between the dense and porous aluminum was the existence of pores and the cell wall surface, the driving force for liquid spreading in the porous aluminum was higher due to the facile spreading of the liquid over the cell wall (see Figs. 3 and 4).
The process of liquid eroding the cell walls could change the cell structure. Preventing the liquid from eroding the cell walls is thought to be difficult as the thickness of the cell walls is smaller than L. Figure 5(a) shows that the thickness of the cell walls is generally below 100 µm. However, as shown in Fig. 7, L in the sintered dense aluminum, which is considered equivalent to the capacity of the liquid to erode the cell walls, exceeds 100 µm within the first few seconds of brazing. Reducing L in the cell walls to less than the cell-wall thickness seems to be effective at preventing liquid eroding the cell walls and evolution of the cell structure.
Grain coarsening occurred due to the presence of the liquid phase as coarser grains were observed in the region into which the liquid spread (Fig. 3 and Fig. 5). Grain coarsening was particularly significant near the bonding interface. Figure 10 shows high-magnification elemental maps of Al, Si, and O in the sintered dense aluminum near the interface after 180 s of brazing. Si and O were distributed along grain boundaries. This result indicates that the liquid, which consisted of the melted Al–Si alloy, filled former powder boundaries because the oxides formed on the initial aluminum powder had remained along the powder boundaries after sintering. The liquid eroding along the former powder boundaries was reported in our previous study,7) where TEM analysis showed that sintered dense aluminum before brazing had no cracks, but oxides were present along former boundaries. After brazing, both Si and O were observed. In addition, Fig. 10 shows grains with a diameter of ∼50 µm in which the liquid filled the grain boundaries. Therefore, grain coarsening was presumed to occur via LFM.
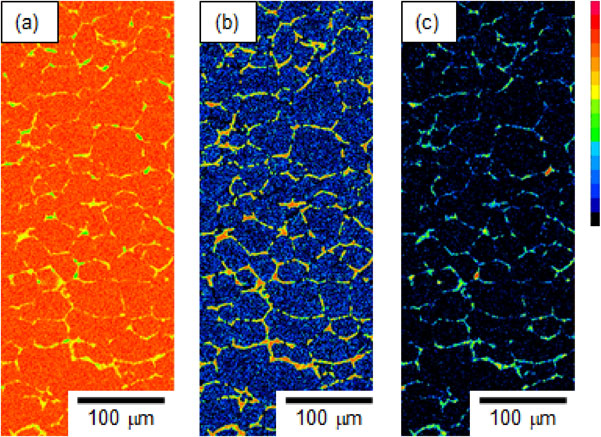
High-magnification elemental maps of (a) aluminum, (b) silicon, and (c) oxygen in the sintered dense aluminum near the interface after 180 s of brazing.
In order to clarify the dominant factor that affected the cell structure evolution, the relationship between the grain diameter and local porosity was investigated. Figure 11 shows P at X = 0.5 mm at various brazing times as a function of G. The value of P at X = 0.5 mm was selected as G was measured near the interface. We observed a linear relationship between P and G, indicating that grain coarsening directly influenced evolution of the cell structure. Furthermore, the line of best fit to the data shown in Fig. 11 is extrapolated to the initial porosity, giving a G value of ∼20 µm, which is equal to the original aluminum powder diameter. This indicates that the grain coarsening is the dominant factor affecting cell structure evolution, because if other stages (spreading, eroding) had as much influence, the extrapolated line would not give the original aluminum powder diameter at the initial porosity. Therefore, suppressing grain coarsening is presumed to be effective at preventing cell structure evolution. For example, using large raw aluminum powder would reduce driving force of the grain coarsening because of large original grains.
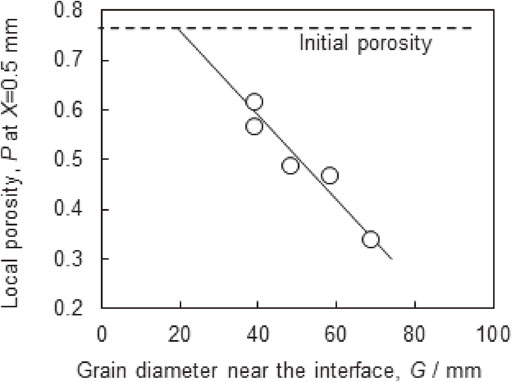
Local porosity (P) at X = 0.5 mm after brazing for various times as a function of the grain diameter near the interface (G).
This study clarified the structural evolution of porous aluminum brazed using an Al–Si-based alloy brazing sheet. The influence of liquid migration and grain coarsening on the cell structure of the porous aluminum was experimentally investigated as a function of brazing time and compared to dense aluminum as a simple model of the cell walls.
We thank Dr. Nishiyama for support with English language during preparation of the manuscript.