2020 Volume 61 Issue 4 Pages 801-804
2020 Volume 61 Issue 4 Pages 801-804
By applying an oscillating potential wave, we obtained an electrodeposited Co–Cu alloy film in which the Co concentration changed periodically at a short modulation wave length. A triangular Co concentration modulation was observed along the film growth direction. The local composition gradient became as high as 50 at%/µm. The Vickers hardness of the composition gradient film was 380 HV. Because this value was much higher than that of simple electrodeposited Co–Cu alloy films, contribution of the composition-gradient structure to hardness was suggested.

Vickers hardness values of the electrodeposited copper, cobalt, Co/Cu multilayer, simple Co–Cu alloys, and composition-gradient Co–Cu alloy films.
The strength of an electrodeposited metallic film can be improved by modification of a microstructure. For example, high strength has been reported in nanograined nickel1,2) and copper3–5) films that were produced by pulsed-electrodeposition techniques. High strength was also achieved in multilayered films consisting of alternate stacks of two dissimilar layers fabricated by electrodeposition6–10) and physical deposition techniques.11–15) To understand the strength increase in a multilayered film, several micromechanisms including dislocation pile-ups at interfaces, a hairpin-shaped dislocation gliding within a component layer, and etc. have been proposed.16) These models assume the existence of a well-defined interface between adjoining layers.
Meanwhile, a diffused interface could likely contribute to a strengthened multilayer film. Oberle et al.9) reported that the annealing at 400°C for 30 min led to an increase in hardness on an electrodeposited Ni/Cu multilayered film, while the hardness values of well-annealed films decreased. The decreased hardness of the well-annealed samples can be understood simply from the unstable structure against heat treatment, because the Ni and Cu layers are easily intermixed at an elevated temperature. However, the increased strength in the shortly annealed sample could not be explained by the strengthening models based on the defined interfaces. In their literature, some interdiffusion between the layers was suggested at the shortly annealed sample. The interdiffused interface should produce a region of gradient composition. Accordingly, the authors conceive the idea that a composition-gradient area in a multilayered film can contribute to strengthening.
A single-bath electrodeposition technique using a rectangle potential wave allows us to produce a multilayered metallic film, owing to the difference in standard electrode potentials between metals contained in electrolyte. However, only limited couples including Ni/Cu,6–10) Co/Cu,17–19) Ag/Pd,20) Zn/Co,21) and etc. have been able to be fabricated. The Co/Cu couple is a representative multilayer that can be obtained by the single-bath technique, as well as the Ni/Cu multilayer. The Co–Cu alloy separates into Co- and Cu-rich solid solutions under equilibrium, while the Ni–Cu and the Ag–Pd alloys are all-proportional solid solutions.22) Hence, in the Co/Cu multilayers, an interdiffused region is hardly formed by annealing, unlike in the multilayers consisting of two elements of the all-proportional solid solution type. Hence, in the present study, we attempted to fabricate a Co–Cu alloy film with high composition gradient only by a special electrodeposition technique in which an applied potential changes continuously. The hardness of the composition-gradient Co–Cu alloy film was measured. The authors compared the hardness value with those of Co–Cu alloy films grown under constant potentials and a Co/Cu multilayered film.
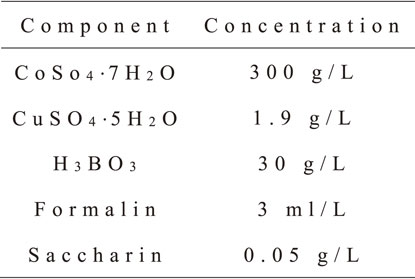
In an electrolytic solution containing two types of metallic ions, the deposit composition depends on the applied potential. In the solution for Co–Cu alloy electrodeposition, the standard electrode potential of cobalt (−0.283 V) is lower than that of copper (+0.340 V).23) In this case, both cobalt and copper deposit simultaneously at a high cathodic potential, while almost pure copper deposits exclusively at a low potential. When the Cu ion content in a solution was considerably lowered in comparison with the Co ion content, a Co-rich alloy was deposited at a high potential (or a high current).17) Hence, we used the solution where the content of copper sulfate was lower than that of cobalt sulfate by a factor of approximately one hundred. The contents of the solution used in the present study are listed in Table 1.
During electrodeposition, the solution was maintained at 313 K and was agitated. A potentiostat (Hokuto-Denko HA-151A) was used to apply a potential. The potential was measured with an Ag–AgCl reference electrode.
A Pt substrate was used for the electrodeposition of the films that were analyzed by X-ray diffraction (XRD) and by energy dispersive spectroscopy (EDS) in a scanning electron microscope (SEM). The electrodeposited films for EDS analysis in a transmission electron microscope (TEM) and for the hardness measurement were deposited on copper substrates that were annealed at 973 K for 1 h in vacuum. All the substrates were covered with lacquer for insulation, except on a circular area (ϕ9 mm) that was exposed in the solution.
In the present electrodeposition, we used three types of potential waveforms, as shown in Fig. 1. At a constant potential corresponding to a conventional electrodeposition (Fig. 1(a)), an electrodeposited film should be a Co–Cu alloy with a homogeneous composition distribution with respect to the growth direction. An electrodeposited Co–Cu alloy film fabricated under a constant potential is denoted as a “simple” Co–Cu alloy film in this study. When applying two different potentials alternately (Fig. 1(b)), we can obtain a Co/Cu multilayered film. Figure 1(c) shows an oscillating potential wave at which the potential changes continuously. Because the deposit composition is sensitive to the potential, a fabricated Co–Cu alloy film can have composition modulation.

Schematic illustrations of potential waveforms for electrodeposition: (a) constant, (b) rectangle, (c) oscillating potential waves for fabricating simple, multilayered, and composition gradient Co–Cu films, respectively.
We first electrodeposited simple Co–Cu alloy films to investigate the dependence of deposit composition on the applied potential. The electrodeposition tests were conducted at twelve constant potentials ranging from −720 to −590 mV vs standard hydrogen electrode (SHE). The thickness of the deposits was 3 µm. The Co compositions of the obtained deposits were analyzed with the EDS system equipped with the JEOL JSM 6500F SEM.
The composition-gradient Co–Cu alloy film was electrodeposited using the same solution. At the beginning of electrodeposition, the applied potential was −655 mV vs SHE. For monitoring film growth, we estimated an instantaneous deposit thickness from a current consumed during 0.01 s. The film growth during this duration was estimated by the Faraday’s law. The current efficiency was assumed to be 75% for the thickness calculation. At every 0.01 s, the potential was adjusted such that the chemical composition changes linearly with respect to thickness, using the potential–Co composition relation that was obtained at the abovementioned experiment. The potential increment (or decrement) direction was inverted when the estimated composition-gradient thickness became 0.5 µm. During the electrodeposition, the applied potential was oscillated approximately within the range from −655 to −625 mV vs SHE. The total thickness of the composition-gradient film was 3 µm.
The microstructure of the composition-gradient Co–Cu alloy film was characterized by XRD using Co-Kα radiation (Rigaku RINT-2200) and by the EDS analysis with TEM (JEOL JEM-2100). To prepare the thin foil, the electrodeposited surface was additionally covered by a thick electrodeposited copper. Subsequently, the sample was machined to a thin disc of 3 mm diameter; the disc surface was perpendicular to the composition-gradient film. The disc was thinned with a dimple grinder and an Ar-ion milling device.
The Vickers hardness of the electrodeposited films was measured with the Shimadzu DUH-W201. The hardness tests were conducted on the samples in which the electrodeposited films were not removed from the copper substrates. The indentation force and the measurement number for each sample were 10 mN and 10, respectively. The hardness measurements were conducted also on the electrodeposited films including the electrodeposited copper, cobalt, Co–Cu alloy, and Co/Cu multilayer. These films had a 3-µm thickness. The individual layer thickness of the Co/Cu multilayer was 0.5 µm.
Figure 2 shows the Co concentrations of the simple Co–Cu films grown under the constant applied potentials ranging from −720 to −590 mV vs SHE. The Co concentration of the simple Co–Cu alloy films decreased with increasing potential. It is thus suggested that the cobalt concentration of an electrodeposited film is adjustable with the applied potential, at the least in this potential range.

Relationship between the Co concentrations of electrodeposited Co–Cu films and the applied potentials that were constant during electrodeposition.
A cross section of the Co–Cu alloy film fabricated by the oscillating potential was analyzed by the EDS in the TEM. Figures 3(a) and 3(b) show the distributions of cobalt and copper, respectively. The intensities of these two elements fluctuated with respect to the growth direction. Point analyses along a line parallel to the growth direction were also conducted on the composition gradient film to quantify Co concentrations. Figure 3(c) shows the Co concentrations, plotted against the position x. The position x = 0 coincides with the original surface of the copper substrate surface. The Co concentration changed periodically and exhibited a triangular form, where the maximum and minimum Co concentrations were approximately 87 at% and 61 at%, respectively. A half wavelength of the periodic change was approximately 0.5 µm. Because of the triangular form, this Co–Cu alloy film can be regarded as alternate stacks of two layers having positive and negative composition gradients. The absolute values of the gradients were approximately 50 at%/µm for both the positive and negative gradients. It is noteworthy that such a high composition gradient cannot be attained in a conventional composition-gradient material at which the concentration changes monotonically throughout the thickness. The present periodic microstructure enabled us to achieve such a high composition gradient in the entire film.

EDX maps of (a) Co and (b) Cu at cross section of the Co–Cu alloy film fabricated by the oscillating potential. A change in Co concentration along the growth direction is shown in (c).
Figure 4 shows the XRD patterns of the composition-gradient Co–Cu alloy, the simple Co–Cu alloy of cCo = 75 at%, and the Co/Cu multilayered film. In the XRD pattern of the multilayered film, two fcc (111) peaks corresponding to the Co and Cu layers are visible. The composition-gradient and the simple Co–Cu alloy films showed single (111) peaks. The fcc (111) peak angle of the composition-gradient film was 2θ = 51.5° and was nearly equal to that of the simple Co–Cu alloy film of cCo = 75 at%. This peak angle is consistent with the EDS analysis that the average Co concentration of the composition gradient film was 74 at%. It is anticipated that the lattice parameter of the composition-gradient film spread over a certain range because the Co composition changed from 61 to 87 at%. However, the composition-gradient film revealed the single peak, which was similar to that of the simple Co–Cu alloy. Hence, one can consider that the lattice parameters were deviated from intrinsic values near the regions with the lower and upper Co concentration peaks.

XRD patterns of the electrodeposited Co/Cu multilayer, simple Co–Cu alloy (cCo = 75 at%), and composition-gradient Co–Cu alloy films.
Typical TEM images of the electrodeposited films are presented in Fig. 5. Figure 5(a) is the microstructure of a Co-rich layer of the Co/Cu multilayers, where the applied potential was constant during the growth of each layer. Elongated grains and equiaxed grains whose sizes (or thicknesses) were typically 10–30 nm are visible. Figure 5(b) is the microstructure of the composition-gradient Co–Cu alloy, where the applied potential changed during the film growth. We can recognize fine grains whose size are 10–20 nm.

Typical dark-field TEM images of (a) the Co-rich layer of the Co/Cu multilayered film with 500 nm layer thickness and (b) the Co–Cu composition-gradient film.
The Vickers hardness tests were conducted on the composition-gradient, simple Co–Cu alloy, Co/Cu multilayer, electrodeposited cobalt and copper films. Three kinds of the simple Co–Cu alloy films electrodeposited at E = −634, −645, and −665 mV vs SHE were prepared for the hardness measurement. These Co concentrations were almost included within the oscillating Co concentrations in the composition-gradient film. Figure 6 shows the Vickers hardness values of these films. It is noted that the measured hardness values should be affected by the soft copper substrate. Hence, in this study, we used a hardness value to estimate the relative strength among the samples. The Vickers hardness values increased in the ascending order of the electrodeposited copper, cobalt, Co/Cu multilayer, simple Co–Cu alloys with the high Co contents, and composition-gradient films. The Vickers hardness of the composition-gradient film was amounted to 380 HV, and this value was certainly higher than those of the Co/Cu multilayered film and the simple Co–Cu alloy films whose hardness values were approximately from 190 HV to 250 HV. Because the Co concentrations of the simple Co–Cu alloy films (cCo = 57, 75, and 85 at%) were almost included in the Co concentration range of the composition-gradient film, the enhanced hardness in the composition-gradient film would not result from solid-solution hardening. Furthermore, no significant difference between grain sizes of the composition-gradient film and the Co-rich phase grown at a constant potential. Accordingly, it was suggested that strength of Co–Cu alloy was possibly enhanced by introducing the high composition-gradient structure. However, the hardening mechanisms have not clarified yet. The microstructure producing the single XRD peak (Fig. 4) may be related to the hardening in the composition-gradient film.

Vickers hardness values of the electrodeposited copper, cobalt, Co/Cu multilayer, simple Co–Cu alloys, and composition-gradient Co–Cu alloy films.
This study was supported by JSPS KAKENHI for Scientific Research in Innovative Areas “MFS Materials Science” (Grant Number 18H05483), and JSPS KAKENHI for Scientific Research (C) (Grant Number 19K05034).