2020 Volume 61 Issue 6 Pages 1143-1148
2020 Volume 61 Issue 6 Pages 1143-1148
This study investigates the interaction between E. coli bacteria and various surface states of Cu. Pure Cu was oxidized at 298 K, 673 K, and 1273 K, and its surface chemical state was characterized using X-ray photoelectron spectroscopy. The oxidized specimens were immersed in both bacteria-free and bacteria-containing solutions, and the release of Cu ions from each specimen was evaluated. As a result, the specimens, which existed as mainly Cu0 or Cu+ on the surface, exhibited the same corrosion behavior and promoted the elution of Cu ions due to the presence of E. coli in the immersion solution. In contrast, the release of Cu ions from the specimen that existed mainly as Cu2+ did not change in the presence of bacteria. According to the XPS analyses before and after immersion in bacteria-free and bacteria-containing solutions, the generation of Cu2+ on the surface was inhibited by the presence of E. coli, and promoted Cu ion elution. This study proposes that the specific corrosion behavior of Cu due to bacteria is beneficial for developing its antibacterial properties.

Biomaterial-associated infections caused by the formation of biofilms on implant surfaces are still a critical issue for implant surgeries.1–4) A recent major strategy for the prevention of initial stages of infection on implant surfaces is the incorporation of antibacterial elements by modifying the surface of implant materials.5–9) Copper (Cu) and its alloys are widely used as antibacterial agents in hospitals and other facilities, owing to their excellent properties against many bacteria, including multi-drug resistance variants.10–15) Recently, Cu has been used to prevent the formation of biofilms;16–23) hence, it is important to understand the thus-called “contact-killing” antibacterial mechanism for the beneficial use of Cu in vivo. Several studies have proved that the interaction between bacteria and Cu surfaces influence their rapid membrane damage, DNA degradation, and less well-defined cell damage.24–29) Moreover, Hans et al.30) reported that there is a strong difference in contact-killing behavior between CuO and Cu2O. CuO inhibited contact killing even more than metallic Cu. In contrast, thermally generated Cu2O was as effective in contact killing as metallic Cu. Elguindi et al. reported that Cu ion influx into bacterial cells was influenced by the control of surface corrosion, contributing directly to bacteria killing.31) These findings indicate that Cu species on the surface and corrosion behavior play a key role in the contact-killing process. Nevertheless, little attention has been given to the effect of bacteria on the corrosion behavior of Cu, which is necessary to understand the contact-killing mechanism.
The purpose of this study was to investigate the corrosion effect and bacterial viability on different surface states of Cu. We oxidized the surface of pure Cu specimens at 298 K, 673 K, and 1273 K, and characterized their chemical states by using X-ray photoelectron spectroscopy (XPS). Each specimen was immersed for 24 h in bacteria-free and bacteria-containing solutions, followed by evaluation of Cu ions release behaviors. The change in Cu specimen proportions before and after immersion was also investigated. In other words, the interaction between bacteria and various surface states of Cu was investigated to help elucidate the mechanism of contact killing by Cu.
A Cu rod (99.99% pure) was cut to prepare disks 8 mm in diameter and 1 mm in thickness. The disks were fixed on the weight with an adhesive. Their surfaces were mechanically polished using #150, #320, #600, and #800 SiC papers, followed by ultra-sonication in acetone and ethanol. These treatments were performed to remove the adhesive. In addition, the passive oxide layers generally grow in the air. Therefore, specimens were then immersed in ultrapure water for 24 h at 298 K to stabilize the passive oxide layer. This treatment is our routine for surface characterization.32,33) They were used as untreated specimens, described previously as oxidized at 298 K.
Each disk was then air-oxidized for 10 min in an electric furnace (AS ONE, IC-1-6033-12) at 673 K and 1273 K. After normalizing at 298 K, each specimen was washed by ultra-sonication in ethanol to remove the oxides separated from the specimen surface.
2.2 XPS measurementXPS was performed using a spectrometer (JPS-9010MC, JEOL, Tokyo, Japan) with a Mg Kα line (energy: 1253.6 eV; acceleration voltage: 10 kV; current: 10 mA). The pressure of measurement chamber was 1 × 10−7 Pa. All binding energies mentioned in this paper are relative to Fermi level. The spectrometer was calibrated against the Au 4f7/2 of pure gold, Ag 3d5/2 of pure silver, and Cu 2p3/2 of pure Cu. The detection angle to the specimen surface was 90°. The binding energies (all binding energies mentioned herein are relative to the Fermi level) were calibrated with the C 1s photoelectron-energy region peak derived from contaminating carbon (285.0 eV). The specimens oxidized at 298 K after 24-h immersion in bacteria-free and bacteria-containing solutions were also subjected to XPS analysis.
To calculate the integrated intensity of peaks, the background was subtracted from the measured spectrum according to Shirley’s method.34) The chemical state changes of Cu were determined by the modified Auger parameter (α′) on the Wagner plot with those of typical Cu compounds. The α′ of each specimen was calculated using the following eq. (1).
| \begin{equation} \alpha' = E_{K}(\text{Cu L$_{3}$VV}) + E_{B}(\text{Cu 2p$_{3/2}$}) \end{equation} | (1) |
Equation (1) contains both kinetic energy of Cu L3VV, EK(Cu L3VV) and binding energy of Cu 2p3/2, EB(Cu 2p3/2). The compositions of the specimens were calculated according to a described previously method.35) Photoionization cross-section of empirical data36,37) and theoretically calculated data38) were used for quantification. In addition, the proportion of (Cu0 + Cu+) and Cu2+ species present on the surface was calculated using the following equations.39)
| \begin{equation} \%(\text{Cu$^{0}$} + \text{Cu$^{+}$}) = (A - (A1_{s}/B_{s})B)/(A + B)*100 \end{equation} | (2) |
| \begin{equation} \%\text{Cu$^{2+}$} = B(1 + (A1_{s}/B_{s}))/(A + B)*100 \end{equation} | (3) |
An anodic polarization (linear sweep voltammetry) was performed using a potentiostat (HZ-7000, Hokuto Denko). Saturated calomel (SCE) and Pt-black electrodes were used as reference and counter electrodes, respectively. The specimens were then fixed with an O-ring onto a polytetrafluoroethylene holder. The area in contact with the test solution was either 0.385 cm2 (7.0 mm in diameter) or 0.353 cm2 (6.7 mm in diameter). Details of the working electrode were as reported earlier.40) A physiological saline (Saline) containing 1-vol% Luria-Bertani broth (LB-Medium, MP Biomedicals, CA, USA) was used as a test solution for anodic polarization measurement. The components of LB-broth are 10 g L−1 tryptone, 5 g L−1 yeast extract and 10 g L−1 NaCl. After immersing the specimens into the test solution at 310 K, the open circuit potential (OCP) was measured for 10 min. Thereafter, the gradient anodic potential was applied at a constant sweep rate of 1 mVs−1 from OCP to 2 VSCE.
2.4 Bacterial cultureEscherichia coli (E. coli, NBRC3972) bacterium was used in this study. This bacterium is basically pathogenic and their biosafety level (BSL) is two. However, the Pathogenic Organisms Safety Management Committee of the Tokyo Medical and Dental University (22012-025 c) approved the experiment. E. coli are normally used in antibacterial property tests as representative gram-negative bacteria, as described in ISO 22196. It is a rod-shaped bacterium commonly found in the gut. A strain was cultured according to its specific requirements, i.e., in LB broth, for 24 h at 310 K under ambient conditions. The optical densities of the bacterial suspensions were measured at 600 nm using an ultraviolet-visible (UV–vis) spectrometer (V-550, JASCO, Tokyo, Japan) to obtain concentrations of 0.9 × 106 colony-forming unit (CFU) mL−1.
2.5 Dissolution test and viable bacterial countsThe specimens were immersed in bacteria-free and bacteria-containing solutions, which consisted of saline and 1-vol% LB broth, and of saline, 1-vol% LB broth, and a 0.9 × 106 CFU mL−1 E. coli suspension, respectively. They were immersed for 24 h in each test solution (5 mL) at 310 K. The concentrations of Cu ions were then measured by inductively coupled plasma-atomic emission spectrometry (ICP-AES, ICPS-7000 ver. 2, Shimadzu Corp., Kyoto, Japan). Additionally, the number of living bacteria in the solutions was measured with a BacTiter-Glo™ Microbial Cell Viability Assay (Promega, Madison, WI, USA), a luciferase-based assay that quantifies the ATP produced by metabolically active cells.
2.6 Statistical analysisAll values are presented as means ± SD, using commercial software KaleidaGraph (Version 4.1.1, Synergy Software, Reading, PA) for statistical analysis. Following multiple comparisons with the Student–Newman–Keuls method41) to assess the data, a one-way analysis of variance (ANOVA) was used considering P < 0.05 to indicate statistical significance.
Figure 1 depicts the Wagner plot of Cu on the specimens oxidized at 298 K, 673 K, and 1273 K based on the Cu 2p3/2 photoelectron peaks and Cu L3VV Auger peaks, and reference plots from Cu (metallic), Cu2O, CuO, and Cu(OH)2 as reported previously.39) The chemical state of an element was determined by comparison of its binding energy with modified Auger parameter values on the Wagner plot. According to the Wagner plot, Auger values obtained from the specimens oxidized at 298 K and 673 K were 1849.3 and 1849.7 eV, respectively, indicating that Cu existed mainly as Cu2O. In contrast, on those oxidized at 1273 K Cu existed mainly as CuO, as their Auger value was 1851.6 eV.

Wagner plot of Cu on the specimens oxidized at 298 K, 673 K and 1273 K based on Cu 2p3/2 photoelectron peaks and Cu L3VV Auger peaks. Each parameter of Cu (metallic), Cu2O, CuO and Cu(OH)2 were plotted according to literature.37)
The relative concentrations of Cu and oxygen and the proportion of Cu species are summarized in Table 1. The concentration of oxygen and the proportion of Cu2+ increased; the concentration of Cu and the proportion of (Cu0 + Cu+) decreased with oxidation temperature. Moreover, the chemical composition and proportion of Cu species on the specimens oxidized at 298 K and 673 K were different despite having the same Auger values.
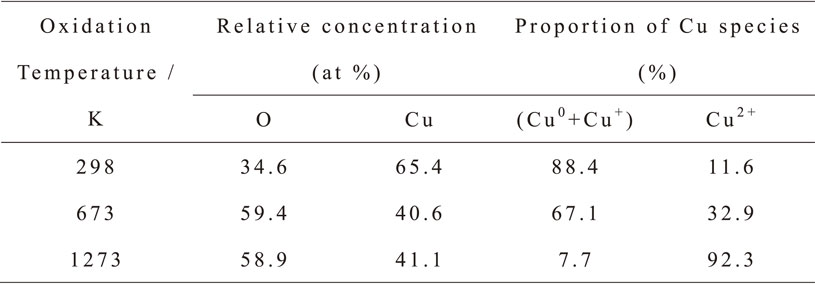
Figure 2 shows the curves for the anodic polarization test results. The curves obtained from oxidized specimens at (A) 298 K and (B) 673 K were almost similar, and from those at (C) 1273 K exhibited a trans-passive state. These results indicate that the corrosion resistances of the specimens oxidized at 298 K and 673 K were lower than that at 1273 K. This reveals the Cu2+ oxide layer of the latter inhibited the elution of Cu ions. Moreover, the corrosion resistances of the specimens oxidized at 298 K and 673 K were almost the same despite the difference of surface composition and Cu species proportion. Generally, the redox potential of Cu+ oxide is relatively higher than that of Cu2+ oxide. Therefore, we believe that the Cu ions elution from the Cu substrate was promoted by the lively movement of electrons from substrate to Cu+ oxide and it led the low corrosion resistance on the specimen oxidized at 673 K.

Polarization curves of the specimens oxidized at (A) 298 K (B) 673 K and (C) 1273 K in a bacteria-free solution at 310 K.
The amount of ions released from the specimens after immersion is shown in Fig. 3(A). Like the anodic polarization measurement results (Fig. 2), the specimens oxidized at 298 K and 673 K showed similar elution behavior. The ions released increased significantly due to the presence of bacteria in the test solutions. On the other hand, specimens at 1273 K had the lowest ion release of all, and it did not change by the presence of bacteria. This result indicates that E. coli led to a Cu corrosion reaction on the specimens oxidized at 298 K and 673 K. Therefore, we believe that Cu0, Cu+ and bacteria interact easily as the proportion of (Cu0 + Cu+) on those specimens was higher than at 1273 K (Table 1). In other words, Cu2+ is stable for bacteria.
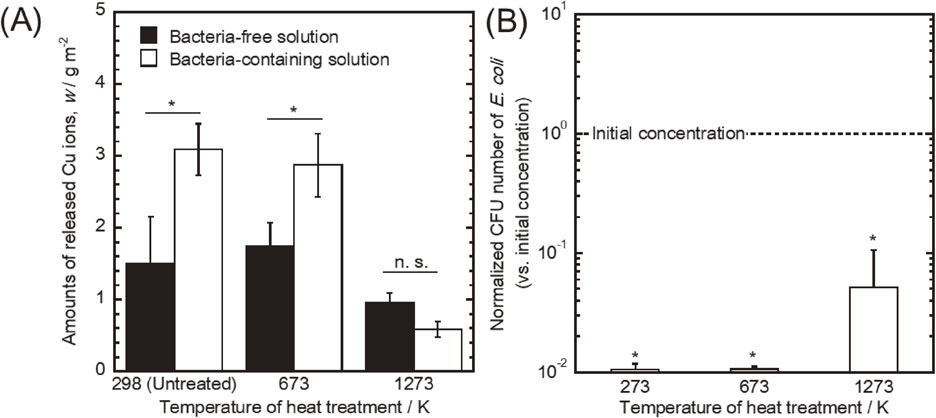
(A) Amount of released Cu ions from the specimens in bacteria-free and bacteria-containing solutions during a 24 h immersion. (B) Number of E. coli in the test solution in which the specimens were immersed during 24 h.
Figure 3(B) shows the normalized CFU counts for E. coli. The vertical axis represents the normalized bacterial number, defined as the bacterial number on each specimen divided by the initial concentration. On all, E. coli numbers decreased significantly compared with its initial concentration. They survived the most on the specimen oxidized at 1273 K. These results are consistent with the report of Hans et al.,30) indicating E. coli were killed by the Cu ions release. Moreover, the numbers of E. coli were dependent upon the amount of Cu ions released. In particular, the specimens that exhibited the elution promotion of Cu ions due to the presence of E. coli developed a high antibacterial effect for it. Therefore, we believe that the proportion of (Cu0 + Cu+) on the surface plays an important role on microbial corrosion and the antibacterial property of Cu. This study proposes the corrosion reaction caused by the interaction among Cu0, Cu+ and bacteria is beneficial for the contact-killing effect. Moreover, several studies have proved that bacteria were killed due to the interaction between Cu and their cell membrane.24–29) Thus, we believe that not only the eluted Cu ions but also Cu0 and Cu+ on the surface interact with the bacteria cell membranes.
Obtained XPS narrow-scan spectra around Cu 2p electron-binding energy regions, from oxidized specimens at 298 K before and after solution immersion, are shown in Fig. 4. The relative concentrations of Cu and oxygen and the proportion of Cu species on the specimens before and after immersion are summarized in Table 2. From Fig. 4, the shake-up peaks appeared more clearly after immersion in each test solution. Moreover, Table 2 shows that the concentration of oxygen increased, and the concentration of Cu decreased after immersion. These results indicate that each specimen surface was oxidized after immersion in each test solution.
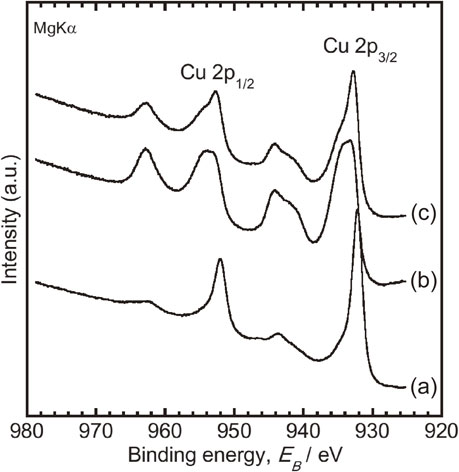
XPS narrow-scan spectra around Cu 2p electron binding-energy regions obtained from the specimens oxidized at 298 K before and after immersion. The spectra obtained from (a) the specimen before immersion, (b) the specimens after a 24 h immersion in a bacteria-free solution, and (c) the specimens after a 24 h immersion in a bacteria-containing solution.

Conversely, the proportion of Cu2+ on the specimen immersed in the bacteria-containing solution was smaller, and the proportion of (Cu0 + Cu+) was larger than in the bacteria-free solution were. Therefore, it is considered that the oxidation reactions after immersion were inhibited in the presence of bacteria. This study revealed that E. coli inhibited the generation of Cu2+. Generally, surface oxides play a key role on metal corrosion resistance. The amount of Cu ions released from oxidized specimens at 1273 K was the lowest of all, and did not promote the elution of Cu ions due to the presence of bacteria (Fig. 3(A)). In other words, Cu2+ is the most stable chemical state for corrosion and bacteria. Moreover, as Cu ions easily interact with the cell membrane,24–29) we think the ions released acted against it. Therefore, we consider that the Cu2+ oxide generation inhibition caused by bacteria led to the elution promotion of Cu ions. In other words, the bacterial effects on Cu corrosion are beneficial to develop the contact-killing process.
The surface Cu0 and Cu+ exhibited the same corrosion behavior and, due to the presence of E. coli, promoted the elution of Cu ions. In contrast, the elution of ions from Cu2+ did not change in the presence of bacteria. According to XPS characterizations, the presence of E. coli inhibited Cu2+ generation and led to the elution promotion of Cu ions.
This study was supported by the Research Center for Biomedical Engineering, Tokyo Medical and Dental University. It was also sponsored by the projects “Creation of Life Innovation Materials for Interdisciplinary and International Researcher Development” and “Cooperative project amount medicine, dentistry, and engineering for medical innovation-Construction of creative scientific research of the viable material via integration of biology and engineering” by the Ministry of Education, Culture, Sports, Science and Technology, Japan.