2020 Volume 61 Issue 8 Pages 1550-1554
2020 Volume 61 Issue 8 Pages 1550-1554
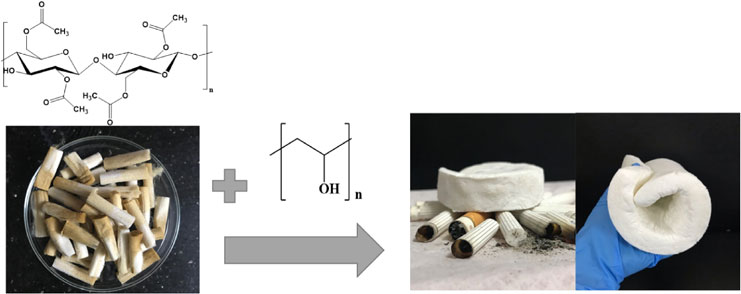
Every year, trillions of cigarette butts (CB) are discarded all over the world without being recycled, thus causing negative impacts on the ecological environment as well as the health of living species. Cellulose acetate (CA), the main component of cigarette butts, is a valuable polymer for numerous applications. For the first time, by using a cost-effective freeze-drying method in the presence of polyvinyl alcohol (PVA) as a cross-linker, high-value engineering cellulose acetate aerogels were successfully fabricated from CB. The effects of component concentrations on the morphology of the CA aerogels were investigated by changing concentration of CA fibers (1–3.0 wt.%) and PVA (0.1–1.0 wt.%). The CA aerogels exhibited an extremely low density (25.4–45.6 mg/cm3), a high porosity (96.46–98.01%), and a low thermal conductivity (0.034–0.039 W/(m*K)). The modified CA aerogels also expressed a great capacity of oil absorption (11.82–25.22 g/g). Hence, the CA aerogel is a promising material for future uses in thermal insulation and oil spill treatment.
Aerogel is an ultra-light solid material that owns industrially useful properties such as low density (0.003–0.500 kg/m3), high porosity (80.0–99.8%), large specific surface area (100–1600 m2/g), and low thermal conductivity.1,2) Consequently, aerogels find prevalent uses in many applications, including thermal isolation and oil spill treatment. It should be noted that many common insulation materials, owing to the low thermal conductivities (0.020–0.044 W/(m*K)), have been widely used in commercial buildings, such as expanded polystyrene, polyurethane, fiberglass, and mineral wool.3) These materials are mostly produced from non-renewable resources. Hence, they could be harmful to the environment.4) It is arguably more beneficial to use alternative eco-friendly materials, such as aerogels, that have the same or sometimes better characteristics to replace the traditional materials.
Cigarette butts are daily discarded all over the world without being recycled, thus causing many bad effects to the environment such as polluting aqua environment and releasing toxic for marine organisms.5,6) Their recycling is thus a must for the better ecosystem. Notably, cellulose acetate (CA) is one of the most important cellulose esters found in cigarette butts as cigarette filters. Studies of CA aerogels have been reported. Some of these works presented disadvantages of the known CA aerogels such as high density (>0.1 g/cm3) and low porosity (<90%).7) Sometimes complicated processes were also required.
Herein we report our attempts to prepare CA aerogels fabricated from cigarette butts. Using a cost-effective freeze-drying method in the presence of eco-friendly chemicals such as polyvinyl alcohol (PVA) as a cross-linker and water as a solvent, CA aerogels were successfully obtained. Our material possesses characteristics that are valuable for uses in oil spill treatment and thermal isolation.
CA fibers were collected from the local tobacco company (Saigon tobacco company) and obtained from wastes of cigarette filters (Degree of substitution (DS) = 2.5). PVA solution was prepared from PVA flakes purchased from HiMedia (India) (MW 160000) and diluted with deionized (DI) water. Methyltrimethoxysilane (MTMS) was purchased from Sigma Aldrich.
2.2 Preparation of recycled CA aerogelsThe raw CA fibers obtained from cigarette filters were first blended to reduce the length of fibers to below 5 mm. Those fibers were then dispersed into the PVA solution that varied concentrations (1–3% w/w). The resulting mixture was homogenized in a probe sonicator for 20 min, followed by being cured at 80°C in an oven for 3 h. These steps were to create a gel with stable three–dimensional (3D) network. The gel was subsequently frozen in a refrigerator at −4°C for at least 24 h before a freeze-drying step to obtain the CA aerogels.
2.3 Development of the hydrophobic CA aerogelsThe CA aerogels were hydrophilic due to the presence of hydroxyl groups in the CA structure and PVA. To form the hydrophobic aerogels that find uses in oil treatment, the above CA aerogels were coated with MTMS. MTMS was chosen for coating because of its low cost and simple procedure for coating. The CA aerogels were placed into an airtight container with an open glass vial of MTMS. The container was then heated in an oven at 70°C for 24 h for the silanization.8,9) The excessive MTMS was then removed in a vacuum oven.
2.4 CharacterizationThe properties of the prepared CA aerogels were characterized by several techniques including scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), thermal gravimetric analysis (TGA), and thermal conductivity analysis. The morphologies of aerogel samples were determined using scanning electron microscopy (SEM) with a JEOL scanning electron microscope operated at 5 kV. The aerogels were characterized using a TENSOR 27 Fourier transform infrared spectroscopy (FTIR, Bruker, Germany) ranging from 4000 to 400 cm−1 at a resolution of 4 cm−1 using KBr disk method.
The density of the CA aerogels was calculated by measuring the weight and volume of the CA aerogel samples. The porosity is determined by eq. (1).10)
| \begin{equation} \varphi = (1 - \rho_{\textit{aerogel}}/\rho_{\textit{bulk}}), \end{equation} | (1) |
Thermal conductivities of synthesized aerogels were measured by using a C-Therm TCi Thermal Conductivity Analyzer (C-Therm Technologies, Canada) in which the modified transient plane source method was applied under an ambient condition. The K-value obtained from the system indicated the thermal conductivity of specimen.11) Samples with different concentrations of CA and PVA were tested three times to determine an average value of thermal conductivity.
The mechanical properties of CA aerogels were determined using an Instron 5500 micrometer (Instron, Norwood, MA, USA). During the experiment, the specimens were placed under a loading rate of 1.0 mm/min.
The oil absorption tests were carried out following a modified ASTM F726-17 standard.12) The aerogels were first weighed, then submerged in a beaker containing 800 mL of 5W-30 oil for 2 h. After the immersion, the aerogels were taken out of the beaker using a stainless-steel mesh basket to remove excessive oil for 0.5 min and reweighed. The maximum capacity of the aerogels for oil absorption was determined using the eq. (2):13)
| \begin{equation} Q_{\textit{max}} = (m_{b} - m_{a})/m_{a}, \end{equation} | (2) |
By using a cost-effective freeze-drying method in the presence of chemicals such as PVA, DI water, the recycled CA fibers (Fig. 1(a)) were successfully converted into the porous CA aerogels (Fig. 1(b)–(c)), as depicted in Fig. 1. The aim of adding PVA into a gel solution of the CA fibers is to form the hydrogen bonding between the hydroxyl groups of the polymer fibers and PVA chains. The mechanism of this formation is illustrated in Fig. 2. In comparison with the formation of cellulose aerogels,14,15) the shape of CA aerogels was more difficult to control because more hydroxyl groups in cellulose chains interacted with the cross linker PVA. Besides, due to the bulkiness of the acetate group in CA, the formation of hydrogen bonding was somewhat limited.
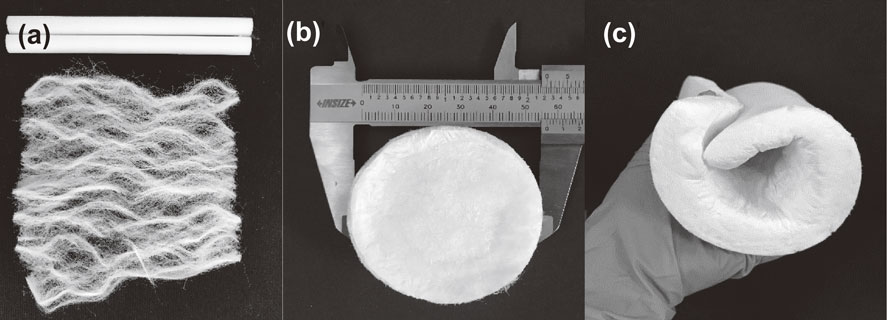
(a) Recycled CA fibers from cigarette filters, (b) the well-controlled shape CA aerogel, (c) the flexible A4-sized CA aerogel.
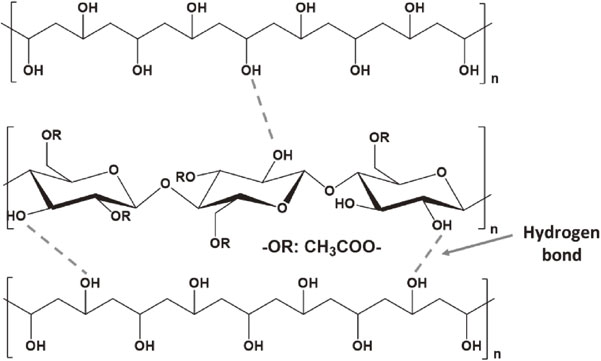
The mechanism of hydrogen bonding formed between CA fibers and PVA crosslinker inside the CA aerogels.
The morphological characterization of CA fibers and CA aerogels by scanning electron microscopy (SEM) is reported in Fig. 3. The fibers had diameters of approximately 35 µm. Figure 3(b) shows how the CA fibers and crosslinker (PVA) performed to generate the structure of aerogels. Moreover, a highly porous structure of CA aerogels could be observed in Fig. 3(c). The aerogel formed from recycled cellulose fibers presented a mesoporous 3D network, as displayed in Fig. 3(b) and 3(c). This could be explained by the large size of recycled cellulose fibers. Comparing the aerogels of CA fibers that were varied in concentrations (Fig. 3(c) and Fig. 3(d)), one can be noticed that the higher the concentration was, the narrower the pore size was and the denser the CA aerogels were. The pore size of the CA aerogels was in the range of 1.35–2.81 nm, indicating that aerogels were both mesoporous and microporous materials.

The SEM images of recycled CA fibers (a), the close up to the pore in the aerogel (b), CA aerogel 1% (% w/v) (c), and CA aerogel 4% (% w/v) (d).
In the next step, the effects of CA fibers and PVA, with regard to different concentrations, on the density and porosity of CA aerogels were determined by changing the concentration of CA fibers from 1% to 3% (wt/vol). As shown in Fig. 4(a), increasing the concentration of CA fibers, which also resulted in mass increments, led to an increase of density of the CA aerogels (from 0.022 g/cm3 to 0.046 g/cm3) and a decrease of their porosity (from 97.52% to 96.39%). Moreover, the more fibers were added, the better well-controlled shape of aerogels would be obtained. Increasing the concentration of the CA fibers could prevent the shrinkage of aerogels during the freeze-drying process, thus maintaining a highly porous structure of CA aerogels.

The effect of concentrations of CA fibers (a) and PVA (b) on density and porosity of CA aerogels.
Furthermore, the presence of PVA in aerogels as a crosslink agent had a great impact on the structure of CA aerogels. As shown in Fig. 4(b), the lower PVA concentration was, the lighter and more porous aerogels could be obtained. When PVA concentration increased, the density of the developed CA aerogels increased (from 0.022 g/cm3 to 0.037 g/cm3). However, a decline in its porosity was witnessed (from 98.28% to 97.04%). A low concentration of PVA could cause the probability of shrinkage that led to the difficulty in controlling the aerogel shape. Besides, the aerogels could be more flexible when low concentration of PVA was used and vice versa. Hence, choosing a suitable concentration of CA fibers and PVA required extreme attention, depending on the expected properties of CA aerogels.
The FTIR results of CA fibers, CA aerogels and MTMS-modified CA aerogels are shown in Fig. 5. The peaks at 3480 cm−1, 2950 cm−1, 1455 cm−1, 1380 cm−1, 1028 cm−1, and 900 cm−1 were all the typical bands of cellulose molecules.16) Moreover, the peaks at 1736 cm−1, 1645 cm−1, and 1163 cm−1 were assigned to the stretching C=O and C–C–O bonds of carboxylic groups.17) Comparing the CA fibers and CA aerogels (Fig. 5(a) and (b)), an enhancement of intensities at peaks 3480 cm−1 and 2950 cm−1, which were assigned to the –OH and –CH2 groups respectively, was observed proving the presence of PVA in the CA aerogels as a crosslinker. Besides, a small increase of intensity at the peak 2361 cm−1 (representing carbon dioxide) was also observed, which could be explained as the good diffusivity and solubility of CO2 in CA.18,19) Comparing the CA aerogels and MTMS-modified CA aerogels (Fig. 5(b) and (c)), a new peak at 775 cm−1 (representing the vibration of Si–C bond) and an increase of intensity at the peak 1270 cm−1 (representing the vibration of –CH3 deformation in MTMS structure) were observed. Overall, these FTIR results confirmed the structures of CA aerogels as well as MTMS-modified CA aerogels.
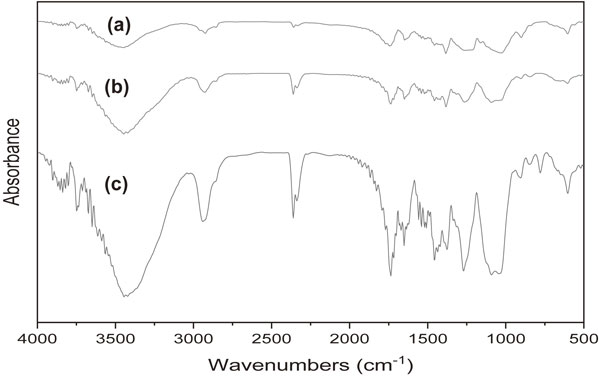
The FTIR spectra of recycled CA fibers (a), CA aerogel (b), and MTMS-coated CA aerogel (c).
For application in thermal insulation, CA aerogels need to have good thermal stability. The thermal profile of the CA aerogel was assessed by thermogravimetric analysis (TGA). The aerogels displayed a typical thermogram with degradation in three steps (Fig. 6(a)). The weight loss below 100°C was attributed to desorption of water on the aerogel. In comparison with other cellulose aerogels, our prepared CA aerogels had smaller weight loss (3.79%) because of the lack of hydroxyl groups thus preventing them from absorbing much water.15,20) The main weight loss of 64.83% starting around 300°C was presumably due to the decomposition of functionalities of cellulose acetate in CA aerogels. Lastly, a loss of 25% starting around 380°C was accounted for the decomposition of polyvinyl alcohol in the aerogel. The results showed that our CA aerogels had a remarkable thermal stability, up to around 300°C, which is similar to other cellulose aerogels.

The thermalgravimetric analysis (TGA) of the CA aerogels (a) and the effect of CA fiber concentration on thermal conductivity of CA aerogels (b).
The thermal conductivity was collected to examine the thermal insulation of CA aerogels. The effect of various fiber concentration in the aerogels on the thermal conductivity is exhibited in Fig. 6(b). The highest thermal conductivity was recorded as 0.039 W/(m*K) at 3% of fiber concentration. Meanwhile, the lowest thermal conductivity was recorded as 0.034 W/(m*K) at 1% of fiber concentration. Moreover, when the fiber concentration increased, the thermal conductivity of CA aerogels increased as well. To compare, the range of this thermal conductivity was as good as that of traditional materials used for thermal insulation such as glass wool (0.031–0.040 W/(m*K)), mineral wool (0.031–0.040 W/(m*K)), expanded polystyrene (0.033–0.040 W/(m*K)), and foamed glass (0.037–0.048 W/(m*K)).21) Hence, the results confirmed that the CA aerogels is a very promising material for application in heat insulation.
3.4 Mechanical propertiesThe compression test is often used to determine the mechanical properties of CA aerogels. The compressive strain-stress curves of CA aerogels with different concentration (1–3% w/v) are depicted in Fig. 7. Table 1 also reports the effect of fiber concentrations on the Young’s modulus of CA aerogels. It could be observed that the CA aerogels exhibited a low range of Young modulus (3.19–27.41 kPa). Indeed, Fig. 1(c) showed that CA aerogel was flexible enough to easily roll up. Increasing concentration of fibers could enhance the Young’s modulus of CA aerogels, since the addition of fibers reinforced the formation of gel structure during gelation. Moreover, the commercial CA fibers used for cigarette filters also had a very small amount of triacetin as a plasticizer to harden the fibers,22) thus also helping to increase the Young’s modulus of the CA aerogels.

The compressive strain–stress curves of CA aerogels with various concentration of fibers.

After being modified with MTMS, the aerogels could become more hydrophobic. The oil uptake of the MTMS-modified CA aerogels made from different concentrations of fibers and PVA is shown in Fig. 8. It is noticeable that the modified aerogel had a great capacity of oil absorption (11.82–25.22 g/g). Furthermore, the increase of the concentration of CA fibers could result in a decrease of the aerogel capacity of oil absorption. The same trend was also observed in the case of PVA concentration. It can be explained based on the properties of CA aerogels. Increasing concentration of PVA and CA fibers would decline the porosity, thus enhancing the density of CA aerogels.
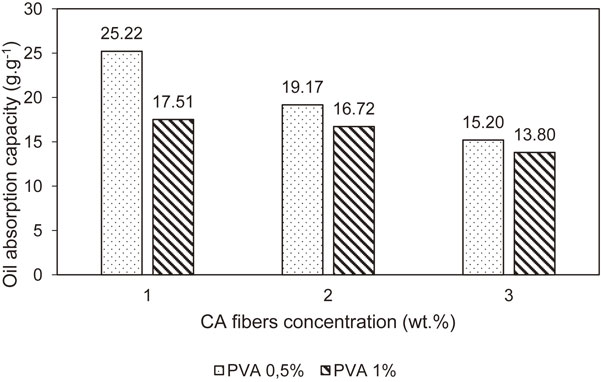
The effect of concentration of CA fibers and PVA on capacity of CA aerogels for oil absorption.
In this report, the CA aerogels were successfully prepared from recycled cigarette wastes using a freeze-drying method in the presence of PVA and water. The obtained aerogels exhibited a high porosity, good flexibility, and excellent mechanical properties. The results offered their potentials to be an excellent material for further applications in the fields of thermal insulation and oil spill treatment.
This research is funded by Ho Chi Minh City University of Technology –VNU-HCM, under grant number T-KTHH-2019-36. We acknowledge the support of time and facilities from Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for this study.