2021 Volume 62 Issue 10 Pages 1533-1540
2021 Volume 62 Issue 10 Pages 1533-1540
We have earlier reported the synthesis of metallic titanium ingot by thermal decomposition process using titanium nitride (TiN) as the intermediate material, with a significant low decomposition temperature of approximately 3500 K. The present study reports the manufacturing process of metallic titanium ingot via thermal decomposition using titanium sulfides (TiSX) as intermediate materials: decomposition temperature of one of the titanium sulfides, TiS, is approximately 4000 K. The thermal decomposition was performed by an arc-flame in a conventional arc melting equipment. The reduced product exhibiting a lustrous surface is analyzed by XRD, and is matched with diffraction pattern of metallic titanium.

Fig. 10 Schematic flowchart of the commercial and presented processes of the metallic titanium ingot manufacturing.
The Kroll method is used in industries for the production of sponge titanium with high purity,1) wherein raw titanium dioxide (TiO2) ore, an upgraded ilmenite, is converted to titanium tetrachloride (TiCl4) in the presence of coke and chlorine gas, followed by a carbochlorination reaction. Then, metallic magnesium (Mg) is used to reduce the TiCl4 intermediate. The metallic magnesium and chlorine gas are electrochemically recycled from the reduction product of magnesium chloride (MgCl2) via electrolysis, thus requiring a large amount of electricity. Moreover, the reaction chamber requires a prolonged cooling time, since the reduction reaction is highly exothermic in nature. Then, Mg and MgCl2 residues are removed via a slow vacuum distillation process, and sponge-like metallic titanium is formed. Finally, the produced sponge titanium is consolidated into the shape of an electrode, and this consumable electrode is melted in an arc melting furnace to form titanium ingot. The conventional process of manufacturing metallic titanium requires complex processes as well as high manufacturing costs.
Owing to the complex industrial manufacturing process, several researchers have focused on exploring novel processing methods for the production of metallic titanium, and recent researches are summarized by Suzuki et al.2) Some researchers have reported the synthesis of metallic titanium particles employing an electrochemical method, with molten calcium chloride or molten salt as the medium,3–6) whereas others have also proposed producing metallic titanium particles through magnesiothermic and calciothermic reductions using molten salts.7,8) In our previous study, we reported a thermolysis (thermal decomposition) method for the production of titanium ingots.9) Titanium nitride (TiN) with a low decomposition temperature was used as the intermediate material. TiN was easily produced from TiO2 in a furnace, under the carbon potential-controlled nitrogen gas atmosphere,10) and also decomposed to metallic titanium ingot instantly by arc-melting, which can easily perform the decomposition temperature immediately. The thermolysis method for production of metallic titanium ingots is a reasonable technique for high-speed and mass production. However, the purity issue of the produced titanium ingots inhibits the replacement of conventional process with the proposed thermolysis method proposed.
The present study also focuses on the thermal decomposition method and uses titanium-based intermediate materials. Sulfur is easily delivered from everywhere and is economically available well. Titanium sulfide (TiSX) is one of the titanium-based intermediates having low decomposition temperature approximately about 4000 K, which is similar to that of TiN (3500 K).11) TiSX is treated as the intermediate material for titanium production, and is already electrochemically performed in molten salt.12,13) Moreover, TiSx can be formed from TiO2 by sulfurization in carbon disulfide gas (CS2) atmosphere,14,15) and it can also be directly formed from TiN in sulfur gas atmosphere.16) The thermodynamic calculations suggest that it is also possible to form TiSx by a carbo-sulfurization reaction of TiO2.11) Hence, the present work proposes one of processing methods for the production of metallic titanium ingot from TiO2 powder. It can be anticipated that this processing technique, based on the chemical conversion of TiO2 to titanium sulfides in sulfur gas atmosphere and its subsequent thermal decomposition to metallic titanium will be an efficient and cost-effective processing method.
Ilmenite (FeO·TiO2), which is a complex titanium-iron oxide ore, is industrially used for the manufacture of metallic titanium, after being upgraded to highly purified TiO2. The bonding strength of titanium and oxygen is significantly high as compared to its bonding with other elements. Figure 1 shows the differences in Gibbs free energy of formation of Ti oxides, nitride, sulfide and chloride by 1 mol gas in the temperature range of 1000–4000 K, found from the thermodynamic data.11) Here, TiO2 is simply regarded as up-graded ilmenite, TiCl4 is commonly used in the industrial production process as an intermediate material to manufacture metallic titanium, and TiN is used as the intermediate material used for the production of metallic titanium ingots using a thermal decomposition method, as shown in our previous report.9) The thermolysis temperature of titanium sulfide (TiS) as one of reference materials of the titanium sulfides is also anticipated from the thermodynamic data (Thermodynamic data of TiS2 for high temperature region is not reported). TiO2 and titanium oxide (TiO) are typically highly stable in the wide temperature range, as demonstrated by their large negative values (Fig. 1) of Gibbs free energies of formation. TiCl4 also has a small negative Gibbs free energy of formation at lower temperature, i.e., 1000 K, but it becomes similar to that of the titanium oxides at approximately 4000 K. In contrast, TiN has a small negative Gibbs free energy of formation in a wide temperature range compared to that of titanium oxides, implying that TiN is easily reduced to metallic titanium as compared to titanium oxides. Moreover, the positive value of Gibbs free energy of formation of TiN at approximately 3500 K indicates its instability above this temperature as it thermolytically separates to form metallic titanium and nitrogen gas. Owing to the small negative Gibbs free energy of formation in a wide temperature range, TiS is also expected to thermolytically separate into metallic titanium and sulfur gas.11) Generally, the thermal decomposition does not depend on the surface reaction. It is not accompanied by a reducing agent such as Mg, and hence, it may expect a faster reaction in the present study.
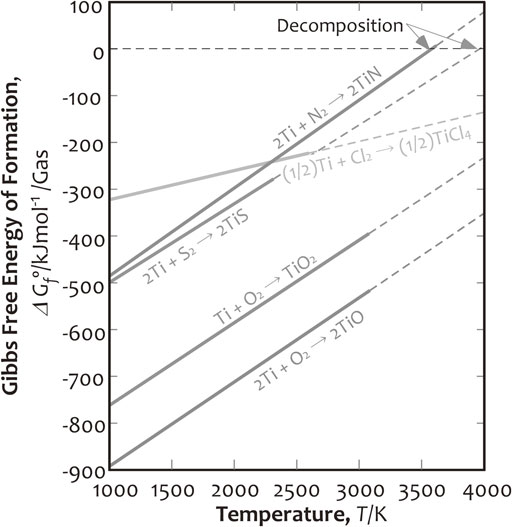
Variation in the Gibbs free energies of formations for the titanium-based compounds.
For the application of this manufacturing process, it is necessary to produce the titanium sulfides as the intermediate materials. The formations of the titanium sulfides are also observed from the Gibbs free energy changes of both the direct and indirect reactions, as shown in Fig. 2. Here, indirect reaction implies combustion with carbon, also known as the carbothermic reaction. As indicated in the Fig. 2, the value of Gibbs free energy of formation is positive for the direct reaction, TiO2 + S2 → TiS2 + O2, which implies that the formation of TiS2 is not possible. However, the negative value of Gibbs free energy of formation for the indirect reaction, i.e., TiO2 + S2 (or 1/2S2) + 2C → TiS2 (or TiS) + 2CO, suggest the formation of TiSX through this process. Here, the titanium oxides undergo multiple phase transformations to form Ti4O7, Ti3O5, and TiO with different degrees of oxidation. The titanium sulfides also form phases with different degrees of sulfurization. Hence, it is necessary to discuss these phases with different degrees of oxidation and sulfurization. A thermodynamic study using potential diagrams is described in the result section to obtain the possibility of thermal decomposition of titanium disulfides to metallic titanium.

Variation in the Gibbs free energies of formation of TiS2 from TiO2 by direct and carbothermic reactions.
Anatase-type titanium dioxide (TiO2) powder of reagent grade (Wako Pure Chemical Industries, Ltd.) was used in the present study. The reported values of thermodynamic properties11) indicate that the anatase-type TiO2 decomposes easily than the rutile type. Hence, anatase-type TiO2 was preferred over rutile TiO2, anticipating a higher reactivity. TiO2 powder was mixed with regent grade graphite powder (Japan Pure Chemical Co., Ltd.), and placed in an alumina reaction boat. The reaction boat was covered with a hemisected carbon crucible and inserted into a quartz tube, as shown in Fig. 3(a). The reaction boat was also connected to a quartz test-tube containing a regent grade sulfur powder (Kishida Chemical Co., Ltd.). The mixture of TiO2 and graphite powder was heated to 1473 K for 7.2 ks (2 h) to 21.6 ks (6 h) inside a horizontal vacuum furnace in an argon-gas atmosphere maintained at an atmospheric pressure of 1 atm. The sulfur powder was also heated from 373 to 773 K stepwise to supply sulfur gas to the mixture of anatase and graphite. The mixture was then rapidly cooled down to room temperature (300 K) under an argon-gas flow with 0.17 ml/s (10 ml/min). The synthesized product was then analyzed using an X-ray diffractometer (XRD) system (MiniFlex II, Rigaku Co.). The outer surface of the product before and after the synthesis is shown in Fig. 3(b).

(a) Schematic of the experimental equipment for the production of TiS2 from the mixture of regent TiO2 and graphite powders, and (b) photograph of the mixture before and after the synthesis.
For the conversion of titanium sulfide to metallic titanium, the synthesized mixture of titanium sulfide powder was inserted into a die with an inner diameter of 20 mm and compressed using an oil-hydraulic cylinder. The compressed mixture of titanium sulfide powder was then heated to 3000–4000 K using a commercial arc melting furnace equipment (DIAVAC Ltd.) under a decompressed argon atmosphere. Here, the decomposition temperature of 3000–4000 K is difficult to perform by a common electric resistance furnace. Melting temperature of crucible made by alumina is about 2300 K and even that of calcia is under 3000 K. Moreover, that temperature is different reacting with the elements of the melts, and its behavior becomes conspicuous depending on combinations of elements. In contrast, the arc-melting technique is easily performed that temperature, and it is unnecessary to consider material of crucibles and its complex combinations. Therefore, the arc-melting technique is selected in the present study. The compressed mixture was subjected to the arc melting several times, with the reaction chamber being cleaned after each step due to the staining caused by dew of sulfur during the process. The resulting products were analyzed using XRD.
Figure 4(a) shows the XRD pattern of the mixture of anatase and graphite powders. The obtained XRD results matches with the standard diffraction patterns of Anatase (PDF #01-075-1537, International Centre for Diffraction Data) and graphite (PDF #01-071-3739, shows in the diffraction pattern of sp19), and was marked in the pattern of the obtained mixture. The diffraction pattern of the mixture obtained after it was heated to 1373 K was matched with the standard data of rutile (PDF #01-071-3739) and graphite (PDF #01-071-3739), as shown in Fig. 4(b). As reported by Kubo and Shinriki,17) the rutile phase is stable above 1473 K, and the anatase TiO2 is transformed to rutile TiO2 at around this temperature. The results shown in Fig. 4(a), (b) are traced well their conclusion. Hence, the reaction process in the present study can be roughly interpreted as the reduction of rutile type titanium oxide to titanium sulfides.

X-ray diffraction patterns of (a) the mixture before the heat-treatment, and (b) that after the heat-treatment at 1373 K for 1.8 ks in pure Ar atmosphere.
Figure 5(a)–(c) shows the XRD patterns of the heat-treated mixture at 1473 K for 7.2 ks (2 h), 14.4 ks (4 h) and 21.6 ks (6 h), respectively. The diffraction patterns presented in Fig. 5(a) match with the standard data of titanium sulfide (Ti1.19S2, PDF #00-041-0930), and the presence of stoichiometric titanium disulfide (TiS2, PDF #03-065-3372) is clearly observed in the XRD patterns (Fig. 5(b) and (c)) of the mixture heat-treated for more than 14.4 ks. Here, Ti1.19S2 is one of non-stoichiometric compounds of TiS2, and its crystallographic and thermodynamic relations are reported.18,19) The titanium-rich non-stoichiometric compound, Ti1.19S2, seems to be prepared by a little lower sulfur partial pressure than the production of TiS2. However, it is interpreted that the presented partial pressure of sulfur is enough for expulsion of oxygen from the TiO2 (convert from TiO2 to TiSx). Although the rutile phase of titanium dioxides (Rutile, PDF #01-071-0650 and PDF #01-071-4809) are seen in the mixture heated for 7.2 ks, they seem to have disappeared from the mixture that was heat-treated for more than 14.4 ks. Graphite (PDF #01-071-3739) is also slightly seen in all the mixtures. Figure 6(a) and (b) shows the diagrams for oxygen partial pressure versus temperature and oxygen versus sulfur partial pressures, respectively. From the relation of oxygen partial pressure versus temperature discussed by Barin and Platzki11) and shown in Fig. 6(a), it is observed that TiO2 is reduced to metallic titanium via Ti4O7, Ti3O5, Ti2O3, and TiO in the wide temperature region including the synthesis temperature of 1473 K. As observed from Fig. 6(b), when the synthesis temperature is fixed/set to 1500 K (i.e., approximately 1473 K), the titanium oxides are converted to the titanium sulfides with a decrease in oxygen partial pressures. The bold lines shown in the diagram represent the results discussed by Barin and Platzki,11) and the weak (gray) lines represent those described by Mills.20) Since the presence of other phases of titanium oxides, such as Ti4O7, Ti3O5, Ti2O3 and TiO, are not confirmed from the XRD analysis, it is expected that TiO2 was directly converted to TiS2 as shown in Fig. 6(b). From the diagram, it is observed that TiO2 is directly converted to TiS2 under the sulfur partial pressure of more than 10−2 atm. Barin and Platzki11) confirm that the sulfur partial pressure becomes less than 10−5 atm. Since the XRD results have shown the presence of TiO2 and TiS2, the thermodynamic relation reported by Barin and Platzki11) can be used in the present study. Here, because the mixture coexists with the graphite powder, as mentioned in the experimental procedure part, the carbon potential is also interpreted to be ac = 1 for the reaction. The equilibrium constant (K) of the reaction, 2CO → C + CO2, as obtained from the literature,11) is given by log K = −3.18 at 1500 K. Thus, CO partial pressure at the situation is determined as PCO = 0.99 atm. From the CO/CO2 ratio, the oxygen partial pressure is also determined to be PO2 = 10−17 atm, and for the reaction CO2 → CO + 1/2O2, the value of equilibrium constant is log K = −5.31 at 1500 K. Since the determined oxygen partial pressure almost matches with that of the value obtained from the diagram shown in Fig. 6(b), we can confirm that TiO2 in the mixture is directly converted to TiS2 as depicted by the arrow of gray in the diagram.

X-ray diffraction patterns of the mixtures after the heat-treatments at 1473 K for (a) 7.2 ks, (b) 14.4 ks and (c) 21.6 ks with sulfurization.

Figure 7(a)–(f) illustrate the decomposition process of TiS2 to metallic titanium ingot. Figure 7(a) shows the initial state of the compressed TiS2 powder of sp29 converted for 21.6 ks, and Fig. 7(b) shows the reduced product exhibiting a lustrous surface. As the decomposition process progresses, rounded products with lustrous surfaces are formed depending on the heating time, as shown in Fig. 7(c)–(e). The sample shown by Fig. 7(a) reached the sample shown by Fig. 7(e) for few minutes except for purging time by argon gas. The XRD patterns of the decomposed product shown in Fig. 7(f) re-named as sp35 and similarly prepared samples (sp26 and sp31 are re-named to be sp38 and sp33, respectively) are shown in Fig. 8(a)–(c). The XRD patterns of the arc-melted sample reacted for 7.2 ks confirm the presence of titanium disulfide (Ti1.19S2, PDF #00-041-0930) and titanium oxides (TiO2, PDF #00-021-1276 and Ti3O5, PDF #00-011-0217) in the product, but there was no trace of metallic titanium in the mixture. However, as shown in Fig. 8(b)–(c), the mixtures reacted for 14.4 and 21.6 ks confirm the presence of metallic titanium (Ti, PDF #00-044-1294) and titanium sulfide (TiS, PDF #03-065-4271) with no trace of titanium oxides in those products. Figure 9(a)–(b) shows diagrams for sulfur partial pressure versus temperature and oxygen versus sulfur partial pressures, as reported by Barin and Platzki11) and Mills,20) respectively. Figure 9(a) shows the relation between sulfur partial pressure and temperature and suggests that TiS2 is directly reduced to metallic titanium above 2000 K. During the reduction process, as the temperature in the arc furnace reaches 4000 K, the sulfur partial pressure decreases to 10−3 atm. Since the sulfur partial pressure is decreased by purging an atmospheric pressure of argon gas, the reduction of TiS2 to metallic titanium is possible at this temperature. Hence, it can be interpreted that the XRD patterns should only contain the phases of metallic titanium. In contrast, since TiS is precipitated at a temperature less than 2000 K, its presence in the XRD results can be interpreted as a precipitated phase during the cooling process after thermal decomposition. Additionally, the ingots synthesized for 14.4 and 21.6 ks contained phases of reduced metallic titanium and TiS, whereas those synthesized for 7.2 ks consisted phases of precipitated Ti3O5. Figure 9(b) suggest that the remaining TiO2 present in the synthesized mixture could not decrease the oxygen partial pressure sufficiently, indicating incomplete reduction of the mixture to metallic titanium. In other words, the synthesized mixture, which was completely reduced to TiS2, decreased the oxygen partial pressure to less than 10−5 atm as boundary of Ti/TiO, hence forming metallic titanium. Since, TiS is precipitated in the reduced ingot after the thermal decomposition, the sulfur partial pressure is also interpreted to be less than 10−9 atm as limit of production of TiS at cooling during the experiments.

Photographs of the reducing mixture by the thermal decomposition using the arc-flame of the arc melting furnace. (a) The compressed TiS2 prior to the reaction of sp29. (b)–(f) Partially thermal decomposed product having a lustrous surface, and renamed to be sp35.

X-ray diffraction patterns of the products after the thermal decomposition by the arc-flame for various synthesis conditions of (a) 7.2 ks, (b) 14.4 ks and (c) 21.6 ks.
Figure 10 shows a flowchart illustrating the conventional and proposed processing method for the manufacturing of metallic titanium ingot. The industrially performed conventional process uses an upgraded rutile, namely concentrated oxide as TiO2, and produces sponge-like metallic titanium via TiCl4 as the intermediate material, which is electrolytically reduced by metallic magnesium. Subsequently, the sponge-like metallic titanium is subjected to vacuum distillation for the removal of Mg and MgCl2, and it forms titanium ingots by an arc melting. However, as both the magnesium and chlorine gas employed in the conventional process are electrochemically recycled, large amount of electricity is required for the electrolysis of MgCl2. Thus, the complex processing method and consumption of large amount of electricity increases the fabrication cost of metallic titanium. The above-mentioned constraints are overcome in the proposed technique, wherein TiO2 is reduced to metallic titanium ingots by thermolysis, with TiS2 used as the intermediate material, although the numerical comparison will be discussed in future. TiS2 is easily produced by the carbo-sulfurization under carbon potential-controlled sulfur gas atmosphere. Moreover, the reduction rate of TiS2 by the thermolysis does not depend on the surface properties, and seems to be fast. Hence, this method makes the fabrication of metallic titanium easy and cost-effective by eliminating certain complex steps, such as the electrochemical reduction of MgCl2 to produce metallic magnesium, vacuum distillation process, and compaction of the titanium sponge.

Schematic flowchart of the commercial and presented processes of the metallic titanium ingot manufacturing.
As mentioned earlier, the quality of produced titanium ingot is also maintained, since there is no trace of titanium oxides in the titanium ingot produced from the mixture heated for more than 14.4 ks. Although the solubility of oxygen in the metallic titanium is 33 at%O (14 mass%O)16) for wide temperature range, the oxygen content of the produced titanium ingot seems to be low, as the diffraction patterns of metallic titanium shown in Fig. 8(b), (c) matches well with the diffraction pattern of the standard metallic titanium, i.e., a remarkable effect causing with much solving impurity elements similar with titanium products reduced from TiN9) is not confirmed. Moreover, an arc-current of 150 A is used for the thermal decomposition of TiS2, which is significantly low than that used in case of TiN (250–300 A).9) As the reaction temperature is low when the arc-current is less, the chances of complete removal of impurities are low. The melting temperature of reduced titanium ingot is confirmed by the phase relations of Ti–S16,21) and Ti–N systems.22) The binary system of Ti–S has a eutectic point, and the melting temperature of reduced titanium decreases on increasing the sulfur content, above the eutectic temperature, i.e., 1485 K for 14 at%S (10 mass%S). In contrast, the binary system of Ti–N has several peritectic points, and the melting temperature of titanium increases on increasing the nitrogen content above the peritectic temperature, i.e., 2323 K for beta-titanium of 9 at%N (2.8 mass%N) and 2623 K for alpha-titanium of 20 at%N (6.8 mass%N). The difference of the arc-current seems to be influenced by the phase-relation as one of reasons. The melting temperature of titanium for the binary system of Ti–O increases depending on the oxygen content, which is 2158 K for 25 at%O (10 mass%O).16) Moreover, from the binary system of Ti–S, it is known that the sulfur is insoluble into the metallic titanium under the eutectic temperature.16,21) The significant low solubility of sulfur is attributed to the precipitation of TiS in the titanium ingot produced by the thermal decomposition process. The partial pressure of sulfur of TiS precipitation is less than 10−9 atm under 2000 K, as shown in Fig. 9(a). It may be possible to decrease the precipitation of TiS if the partial pressure of sulfur is further decreased (less than that in the present), with purging of atmosphere gas after the reaction in the reaction chamber by inert gas such as argon, but it is difficult to completely avoid the precipitation of TiS as it disperses as a eutectic structure. It may be possible to avoid precipitation and thus decrease the sulfur content from the reduced metallic titanium, through heat treatment, but it could not be achieved in the present study due to the limitations of the equipment. However, considering the characteristic phenomena of the Ti–S system, the treatment of TiS2 as the intermediate material for thermal decomposition process is favorable for the issues.
As mentioned earlier, the proposed method for the production of titanium ingot includes several impurity issues, but the results obtained indicate the separation of metallic titanium. However, extensive investigation and experiments, including quantitative investigation of the impurity elements are necessary for the clarification of the details of this approach via TiS2 as the intermediate material, and are oriented in further studies.
The proposed manufacturing process for reduction of TiO2 to metallic titanium by the thermal decomposition using TiS2 as the intermediate material was experimentally performed. The synthesized TiS2 was easily decomposed with a lower arc-current than that of previously reported TiN. Moreover, TiS2 was easily synthesized by the carbothermic reaction in sulfur atmosphere at a reasonable temperature above 1473 K, which was lower than that of TiN in the previous study. The equipment for the synthesis of mixture, i.e., the conventional electric resistance furnace accompanied with sulfur gas generation system, was easily available. The subsequent decomposition of TiS2 was also performed in an electric arc furnace, and the produced metallic titanium had a lustrous surface. The phases of the synthesized mixture and the thermally decomposed titanium ingot were confirmed through XRD analysis, and were target phases.
This study was supported by the Wesco Scientific Promotion Foundation for the study of the fundamental synthesizing process of titanium sulfide, the Mukai Science and Technology Foundation for the production of high purity titanium sulfide for the novel manufacturing process of metallic titanium, and a cooperative program (202011-CRKEQ-0003) of the Cooperative Research and Development Center for Advanced Materials, Institute for Materials Research (CTDAM-IMR), Tohoku University for the investigation of the decomposition process of titanium sulfide to metallic titanium.