2021 Volume 62 Issue 11 Pages 1632-1638
2021 Volume 62 Issue 11 Pages 1632-1638
Cobalt carbide (Co2C) nanoparticles were synthesized simply in liquid phase using Co(II) acetylacetonate and oleylamine under reflux conditions. Even in the presence of carbon black XC-72, Co2C nanoparticles were formed and simultaneously deposited on the carbon surface. The thin layer consisting of cobalt (oxy)hydroxide was present on the outermost surface of the thus-prepared Co2C nanoparticles, which was confirmed by X-ray photoelectron spectroscopy, but the redox treatment using a cyclic voltammetry technique gradually removed such by-product phase. The resulting Co2C nanoparticles supported on XC-72 exhibited high durability for the oxygen reduction reaction.
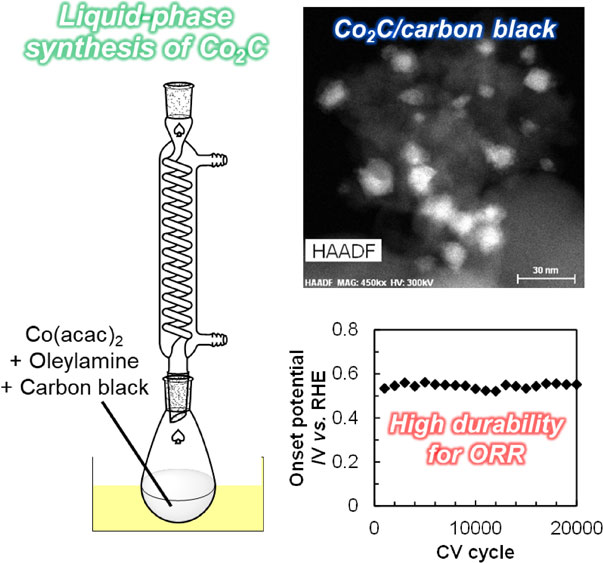
Metal carbides are attractive functional materials and have been investigated in a variety of research fields thus far due to their chemical, physical, magnetic, and electronic properties.1–9) Among various metal carbides, cobalt carbide (CoxC, x = 2 or 3) nanoparticles have been mainly studied as magnetic materials due to their permanent magnetic properties,10–17) while they have also been reported to exhibit intriguing catalytic performance for Fischer–Tropsch synthesis,18,19) dehydrogenation,20) and hydrogen evolution reaction.21) Although CoxC nanoparticles show such attractive properties and applications, their preparation sometimes requires special equipment for chemical vapor deposition22) or electric arc discharge23) and also necessitates highly toxic CO as a carburizing agent.18,24,25) In this respect, the development of simple liquid-phase synthesis methods to obtain CoxC nanoparticles is highly desired. The heat-treatment for cobalt salt (e.g., cobalt acetate) in a solvent with a high boiling point has been developed for synthesizing CoxC nanoparticles.12–16,21,26–30) In these liquid-phase synthesis methods operated at high temperatures, Co2+ is initially reduced to Co0, and a carbon-containing counter anion of cobalt salt is decomposed and leaves carbon atoms on the surface of Co0. Such carbon species adsorbed on Co0 are subsequently taken as a carbon source to form carbide species. In this respect, a source of cobalt is of importance for the liquid-phase synthesis of CoxC. Solvents and additives also play significant roles in the liquid-phase synthesis of CoxC. For example, in the extensively-studied method so-called polyol process, a typical solvent tetraethylene glycol behaves as a reducing and capping agent that controls the rates of Co2+ reduction and particle growth in addition to as a reaction medium.13,31) In some cases, alkali-metal hydroxide or halide was added into the synthetic systems also as a capping agent to adjust those rates.13,16,21) These factors indeed impacted on the resulting CoxC phase(s): single-phase Co2C,13,21,27,29) single-phase Co3C,14,28,30) and a mixture of Co2C and Co3C.12–16,26) These facts motivated us to investigate the liquid-phase synthesis using a different combination of cobalt source and solvent. In this manuscript, the combination of Co(II) acetylacetonate (Co(acac)2) and oleylamine was found to form Co2C nanoparticles in the presence/absence of a carbon black support, which enables their use as an electrocatalyst. The catalytic performance of the carbon black-supported Co2C nanoparticles for oxygen reduction reaction (ORR), which is an important half reaction for fuel cell systems and thus one of the key technologies to accomplish the Sustainable Development Goals (SDGs) adopted by the United Nations, were examined by electrochemical techniques.
Cobalt carbide nanoparticles were synthesized in liquid phase by modifying our previously reported procedure for nickel carbide nanopaticles,32) as described below. All reagents were used as received unless otherwise specified. In a 300 mL egg-plant flask equipped with a reflux condenser, 129 mg (0.5 mmol) of Co(acac)2 (Sigma-Aldrich) was dissolved into 50 mL of oleylamine (Sigma-Aldrich). The mixture was initially stirred at 50°C for 30 min and subsequently refluxed at 320°C for 30 h in a mantle stirrer. The mixture was cooled to room temperature, and then n-hexane (FUJIFILM Wako Pure Chemical) was added to collect the suspension. The solid and liquid phases were separated via filtration and washed with n-hexane three times in the same manner. After drying at 60°C overnight, the black powder was obtained. Hereafter, to clearly distinguish this specimen from general Co2C compounds, the sample prepared via this liquid-phase (LP) method was denoted as Co2CLP.
2.2 Liquid-phase synthesis and simultaneous deposition of cobalt carbide nanoparticles over carbon black supportCobalt carbide nanoparticles supported on carbon black Vulcan XC-72 (Cabot, specific surface area 250 m2 g−1, denoted as XC), which has been widely examined for catalytic reactions,33–37) were prepared via almost the same procedure described above. In a 300 mL egg-plant flask equipped with a reflux condenser, 129 mg (0.5 mmol) of Co(acac)2 was initially dissolved into 50 mL of oleylamine. Then, 50 mg of XC, which was treated at 500°C for 2 h under N2 flow prior to use, was dispersed into the mixture. Following ultrasonication for 30 min to disperse the XC particles, the suspension was stirred and refluxed at 320°C for 30 h in a mantle heater. Subsequently, the mixture was cooled to room temperature, and then n-hexane was added. The solid and liquid phases were separated via centrifugation at 15,000 rpm for 15 min followed by decantation. In the same manner, the solid phase was washed with n-hexane three times. After drying at 60°C overnight, the black powder of the XC-supported cobalt carbide nanoparticles, denoted as Co2C/XC, was obtained.
2.3 CharacterizationThe crystalline phase of the cobalt carbide nanoparticles was investigated by powder X-ray diffraction measurement (XRD; Ultima IV, Rigaku, equipped with a semiconductor detector, Cu Kα radiation at 40 kV and 40 mA). The particle size and dispersion of elements were analyzed using scanning transmission electron microscopy (STEM; Titan™ 60-300 Image Corrector, Thermo Fisher Scientific) in the high-angle annular dark-field (HAADF) mode, equipped with an energy dispersive X-ray spectroscopy system (EDS; AMETEK). The surface-averaged diameter davg was calculated from Σnidi3/Σnidi2, where di is the diameter of particle i and ni is the number of particles with the size of di. The surface state was examined by X-ray photoelectron spectroscopy (XPS; PHI 5600, ULVAC-PHI, monochromatized Al Kα radiation) with Ar ion etching at 3 kV and 25 mA of emission current. Prior to the XPS measurement, each sample was fixed on an In foil.
2.4 Elucidation of catalytic performance for oxygen reduction reaction (ORR)A rotating disk electrode (RDE) was prepared as follows. The powdery Co2C/XC sample was dispersed in 1-propanol (FUJIFILM Wako Pure Chemical) to prepare a suspension with a nominal solid content of 4 mg mL−1, followed by ultrasonication for 30 min. This suspension was mixed with the same volume of a Nafion dispersion, which was prepared by mixing Nafion 5 mass% dispersion (Sigma-Aldrich) and 1-propanol (respective volume ratio 9:1). Then, 10 µL of this mixture was dropped onto a rotating disk (ϕ5 mm) and dried. The performance and durability of the prepared RDE were evaluated by linear sweep voltammetry (LSV) and cyclic voltammetry (CV), where a three-electrode system (ALS, RRDE-3A, equipped with an electrochemical analyzer) employing a working electrode (the prepared RDE), a counter electrode (Pt mesh), and a reference electrode (Ag/AgCl) was used. Prior to each test, the electrolyte consisting of 0.1 M HClO4 (FUJIFILM Wako Pure Chemical) was saturated with O2. For the LSV and CV measurements, the typical rotation rate of the RDE and the range of operating voltage were 1,600 rpm and −0.1 to 1.2 V, respectively. The sweep rates for the LSV and CV measurements were 10 and 100 mV s−1, respectively. After the 20,000th CV cycle, the electrolyte solution was analyzed by inductivity coupled plasma-atomic emission spectroscopy (ICP-AES; SPECTRO ARCOS, AMETEK) to determine the amount of Co species leached from the Co2C/XC catalyst.
To investigate whether cobalt carbide was formed in a liquid phase, Co(acac)2 was treated at 320°C in an oleylamine solvent under the reflux conditions (see Experimental), where the acetylacetonate ligand is expected to be decomposed and leave carbon atoms that are used as a carbon source to form carbide species as reported in previous liquid-phase synthesis systems.12–16,21,28–30,38) The XRD pattern of the resulting black powder is shown in Fig. 1 (see the diffractogram for Co2CLP). The prominent diffraction peaks were observed at 37.0°, 41.3°, 42.6°, 45.7°, and 56.7°, all of which were attributed to Co2C, while no other peaks originated from another known cobalt carbide phase (i.e., Co3C) were detected. This observed pattern demonstrated that this liquid-phase synthetic system produced Co2C only, without any other phases. Considering that reported liquid-phase synthesis operated in various solvents such as tetraethylene glycol,12–16,30) octadecylamine,28) and a mixture of tetraethylene glycol and oleylamine38) with/without additives produced a Co3C phase or a complicated mixture (consisting of CoO, Co2C, and Co3C) instead of Co2C, the choice of solvents and additives significantly impacts on the resulting phase(s). In addition to these diffraction peaks relating to the Co2C phase, the halo at ca. 22°, which was possibly derived from an amorphous material, and the other peak at 44.3°, which was assignable to graphite, also appeared. In the HAADF-STEM image shown in Fig. 2, small particles with the surface-averaged diameter (davg) of 7.4 nm were observed, along with cloudy amorphous material. The agreement of d-spacing values elucidated from the expanded HAADF-STEM image with the XRD pattern identified the nanoparticles observed in the HAADF-STEM image surely as Co2C. The elemental mapping for cobalt, carbon, nitrogen, and oxygen atoms by EDS revealed that cobalt and oxygen atoms were located at the same place, suggesting that the surface of Co2C nanoparticles was oxidized (vide infra). Meanwhile, carbon and nitrogen atoms were found in the whole image, suggesting that the cloudy amorphous solid material was originated from oleylamine. These elemental mapping images combined with the XRD data in Fig. 1 indicated that an amorphous carbon material with a slight amount of graphite was formed from oleylamine. We infer from this indication that oleylamine underwent the oxidation to leave carbon atoms, which subsequently participated in the formation of carbonaceous materials; in other words, oleylamine possibly acted as not only a solvent but also a reducing agent, similar to previously reported systems using tetraethylene glycol.13,31)
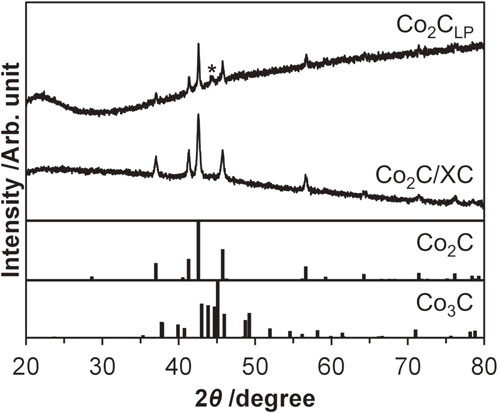
Crystal structure of cobalt carbide nanoparticles synthesized in liquid phase in the absence/presence of carbon support XC. The peak marked with an asterisk is derived from graphite (PDF Card #75-1621). References: Co2C (PDF Card #04-004-4639) and Co3C (PDF Card #01-089-2866).

Morphology of Co2CLP and element distribution: (A) HAADF-STEM image, (B) expanded HAADF-STEM image, and (C–F) elemental mapping images by EDS.
The Co2CLP sample was further analyzed by XPS combined with Ar ion etching technique (Fig. 3). The pristine Co2CLP sample without etching provided no peak in the Co 2p XPS spectrum but exhibited peaks in the C 1s, N 1s, and O 1s regions. The observed peak in the C 1s XPS spectrum was derived from sp2-C atoms in carbonaceous materials (centered at 284.5 eV)39,40) and in amine species (ca. 286 eV).40) It should be noted that since an In foil was used to fix the Co2CLP sample, this peak was not from carbon tape, which is typically used in XPS. In the N 1s XPS spectrum, the broad peak at ca. 399 eV was attributed to N atoms in amines.41) The peak at 531.3 eV in the O 1s XPS spectrum, which was possibly due to O atoms from (oxy)hydroxide species.42) After the first etching process, the Co 2p1/2 and Co 2p3/2 peaks appeared at 793.5 and 778.5 eV, respectively, with satellite peaks at ca. 796 and 782 eV. These peaks demonstrated the presence of Co carbide species,21,25) in good agreement with the results of XRD and STEM (vide supra). Although the Co 2p XPS spectra thus revealed the formation of Co carbide species, in the C 1s XPS spectra, the peak assignable to carbidic C atoms, which is known to appear at ca. 283 eV,25,43) was not clearly observed because of the overlap with the prominent sp2-C-derived peak. In stark contrast to Co and C, almost no peak was found in the N 1s or O 1s regions after the first etching process. These XPS data indicated that the surface of Co2C nanoparticles was covered with residual oleylamine and carbonaceous materials and also oxidized to some extent to form (oxy)hydroxide. Altogether, although residual oleylamine and some by-products were present on the surface, Co2C nanoparticles were successfully synthesized without the formation of another CoxC phase (i.e., Co3C) via the liquid-phase method.
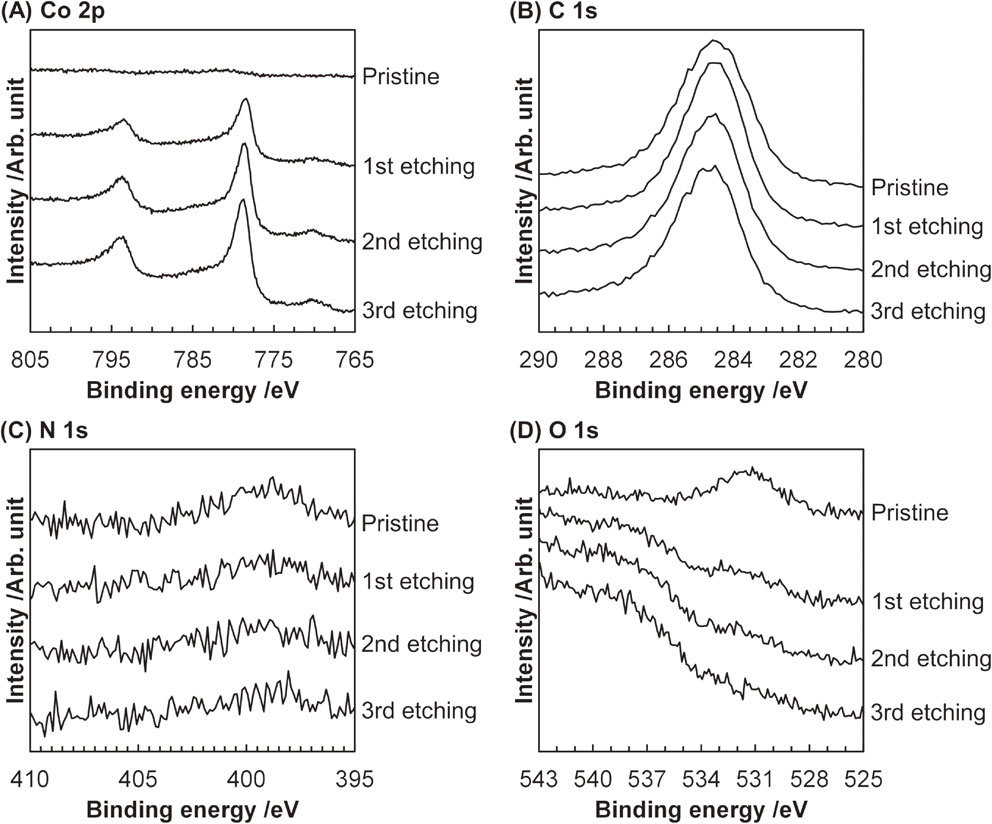
Co 2p, C 1s, N 1s, and O 1s XPS spectra for Co2CLP with Ar ion etching.
These results motivated us to apply this liquid-phase synthesis method to the formation and simultaneous deposition of Co2C nanoparticles over the surface of carbon black XC, since supported nanoparticles are attractive heterogeneous catalysts that exhibit excellent activity for various reactions and also ease of separation from reaction mixtures.44) The XRD pattern of the thus-prepared Co2C/XC is shown in Fig. 1. The pattern given by Co2C/XC was in good agreement with those for Co2CLP and reference, without any other peaks; therefore, Co2C nanoparticles were successfully formed even in the presence of XC in the liquid-phase synthetic system. The HAADF-STEM and elemental mapping images for Co2C/XC are shown in Fig. 4. The small Co-containing particles with davg = 18 nm, which was 2.4-fold larger than the case of Co2CLP (davg = 7.4 nm, vide supra), were observed with the carbon black support, indicating that XC did not inhibit the formation of CoC2 nanoparticles but rather led to their aggregation during the liquid-phase synthesis. The EDS elemental mapping also revealed that O atoms were present at the same place as Co atoms, due to the same reason as the Co2CLP sample (i.e., the surface of Co2C particles was oxidized; vide infra). Note that N atoms were not detected by the EDS mapping analysis for Co2C/XC possibly due to the low N content in the sample.
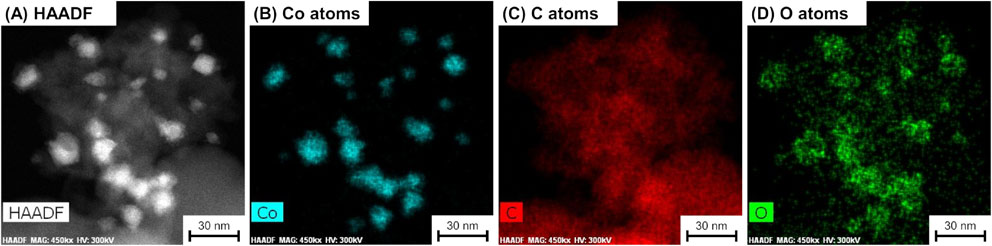
Morphology of Co2C/XC and element distribution: (A) HAADF-STEM image and (B–D) elemental mapping images by EDS. Note that N atoms were not detected for Co2C/XC.
The XPS data for Co2C/XC upon Ar ion etching were shown in Fig. 5. The pristine Co2C/XC sample without Ar ion etching did not provide any peaks in the Co 2p XPS spectrum, but after the first etching process, the sample gave the Co 2p1/2 and 2p3/2 peaks at 793.5 and 778.5 eV, respectively, both of which are characteristic of cobalt carbide species.21,25) In contrast, in the O 1s XPS spectra, the peak at 531.5 eV, which was assignable to O atoms involved in (oxy)hydroxide species,42) originally found for the pristine Co2C/XC sample disappeared after the first etching process. These changes observed in the Co 2p and O 1s XPS data upon Ar ion etching indicate the formation of Co2C nanoparticles covered with thin cobalt (oxy)hydroxide layers, consistent with the case of Co2CLP (vide supra). The C 1s XPS spectra were unchanged and gave a prominent peak at 284.5 eV, which was due to sp2-C atoms,39,40) even after surface etching because this Co2C/XC material mainly consisted of the carbon black support XC. Likewise, no peak was observed in all N 1s XPS spectra, possibly due to the low N content as discussed above.
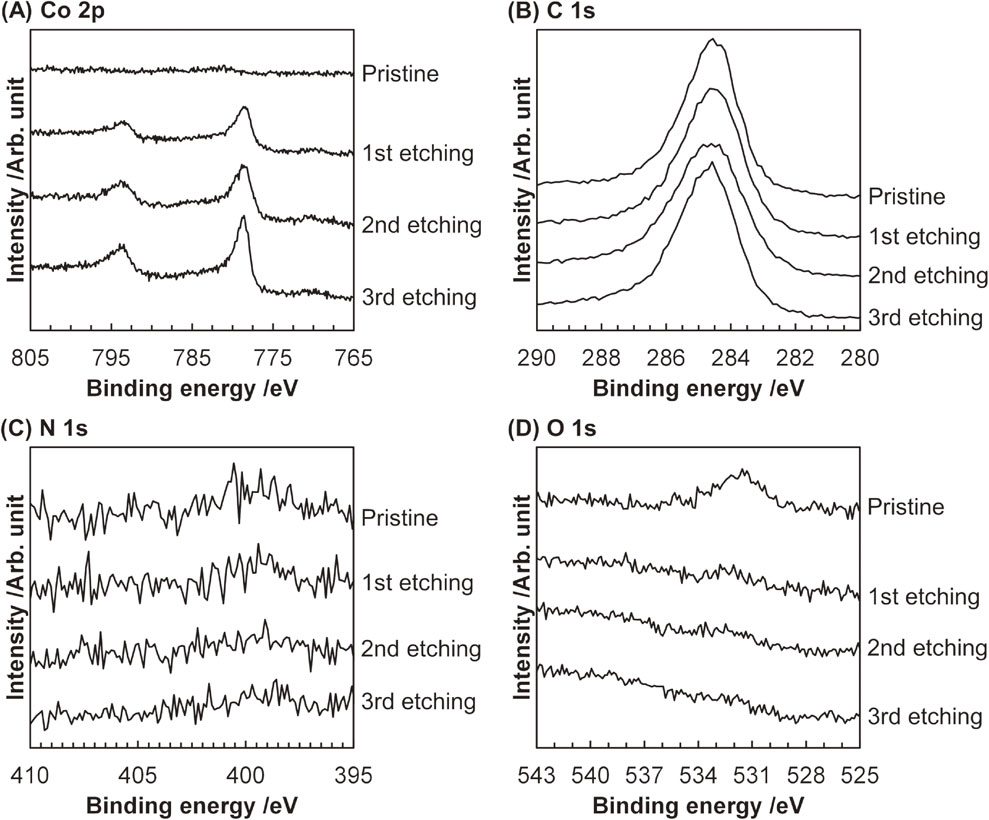
Co 2p, C 1s, N 1s, and O 1s XPS spectra for Co2C/XC with Ar ion etching.
Previous reports predicted that the values of density of states (DOS) for CoxC are similar to that of Pt,45–47) which has been widely investigated as an electrode catalyst for ORR48–50) but desired to be replaced with inexpensive metals. This similarity in DOS values suggests that CoxC species have a potential to be non-Pt ORR catalysts. These reports thus motivated us to examine the ORR performance of the Co2C/XC sample. Figure 6 represents the LS voltammograms for the Co2C/XC catalyst after certain CV cycles. After the first CV cycle, the current density was negligible and the onset potential could not be determined, but the current density gradually increased upon an increase of CV cycles to 2,000 times. Considering that the surface of the Co2C nanoparticles was covered with cobalt (oxy)hydroxide species (vide supra), such undesired phase inhibited the ORR after the first CV cycle but was gradually removed by the subsequent CV treatments, resulting in the exposure of desirable Co2C phase to exhibit the ORR activity. The further increase in the CV cycle did not alter the current density significantly. Except for the LSV measurement after the first CV cycle, the onset potential was kept unchanged even after the 20,000th CV cycle. The average onset potential given by Co2C/XC in these experiments was 0.55 V vs. the reversible hydrogen electrode (RHE). These data clearly demonstrated Co2C/XC to be a highly durable ORR catalyst. The ICP-AES measurement for the electrolyte solution after the 20,000th CV cycle indeed confirmed that the amount of Co species leached from the catalyst surface was negligible (<0.1 ppm). As a reference, a commercially available carbon-supported Pt catalyst (Pt/C, Pt 20 mass%, Sigma-Aldrich) was tested during 10,000 CV cycles (Fig. 6B). The onset potential of Pt/C gradually decreased from 0.64 V vs. RHE, the value of which was lower than the reported values (ca. 1 V)51) possibly due to uncontrolled size of Pt particles, to 0.48 V vs. RHE upon an increase of CV cycles. Therefore, the Co2C/XC catalyst was less active but exhibited higher durability for ORR, compared to Pt/C. Such high stability of the Co2C/XC catalyst would be beneficial to its practical applications, where a low but constant potential is required. To enhance the activity of Co2C-based catalysts, as reported for other materials employed for relating electrocatalytic reactions,52–54) doping of transition metals into Co2C nanoparticles would be a promising approach.
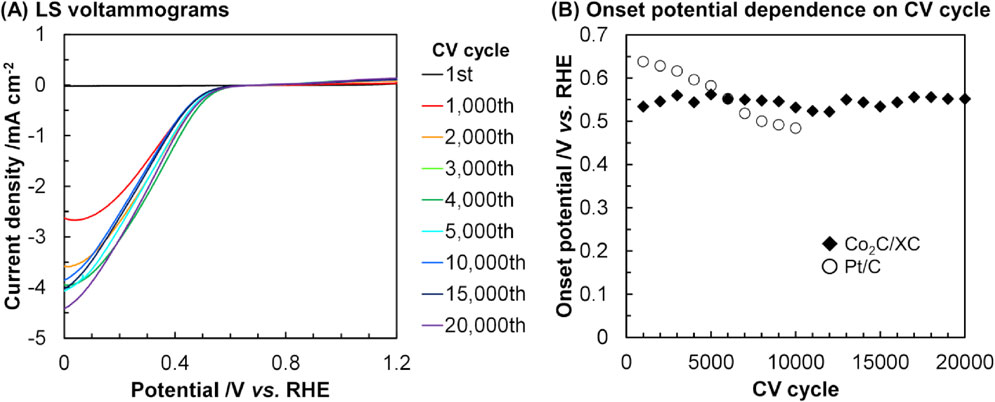
ORR performance of Co2C/XC catalyst: (A) LS voltammograms after designated CV cycle(s) and (B) dependence of onset potential upon CV cycles.
The slope of the Koutechký–Levich plot in Fig. 7 indicated that the ORR over the Co2C/XC catalyst proceeded via the four-electron reduction of oxygen to water, which is the same pathway as the ORR using conventional catalysts,55,56) instead of the undesirable two-electron reduction pathway through H2O2 formation that lowers the ideal onset potential. The overpotential—the difference between the observed onset potential (0.55 V vs. RHE) and the ideal value for the four-electron reduction of oxygen (1.288 V vs. RHE at pH = 1)57)—might be able to be improved by precisely controlling the size of Co2C nanoparticles, similar to the case of Pt-based electrode catalysts.58)

Koutecký–Levich plot for the ORR using the Co2C/XC catalyst at the 10,000th CV cycle. The detailed information is available in Appendix.
The liquid-phase synthesis method treating Co(acac)2 in oleylamine under reflux conditions in the presence/absence of a carbon black support XC successfully provided Co2C nanoparticles without the formation of another cobalt carbide phase (i.e., Co3C). Although the impurity phase consisting of cobalt (oxy)hydroxide was present on the surface of Co2C nanoparticles and did not exhibit any catalytic activity for the ORR, such phase was gradually removed upon the CV cycles. The resulting Co2C nanoparticles supported on XC maintained the onset potential and current density even after the 20,000th CV cycle, indicating a high durability of the Co2C/XC catalyst for the ORR. Although the ORR activity of the Co2C/XC catalyst needs to be further improved, its noble metal-free composition and high durability might be beneficial for practical applications.
The second and the third authors contributed equally to this work. This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI, 24360330) from the Japan Society for the Promotion of Science (JSPS). We appreciate Mr. Yuichiro Hayasaka (The Electron Microscopy Center, Tohoku University) for his support with STEM-EDS analysis.
From the slope of Fig. 7 and eq. (A1) (i.e., Koutecký–Levich equation), the number of electrons which participated in the ORR over the Co2C/XC catalyst was evaluated to be 4.09.
| \begin{equation} \frac{1}{I} = \frac{1}{I_{\text{K}}} + \frac{1}{B_{\text{L}}\omega^{\frac{1}{2}}} \end{equation} | (A1) |
| \begin{equation} B_{\text{L}} = -0.620nFAD^{\frac{2}{3}} v^{-\frac{1}{6}}C \end{equation} | (A2) |