2021 Volume 62 Issue 12 Pages 1681-1687
2021 Volume 62 Issue 12 Pages 1681-1687
In this paper, AlxCoFe1.9Ni2.1 (x = 0.5, 0.8, 1.0, 1.5) high entropy alloys are prepared by vacuum arc melting furnace. The effect of aluminum content on the microstructure and mechanical properties of AlxCoFe1.9Ni2.1 high entropy alloys was studied. The microstructure of high entropy alloy was characterized by SEM, X-ray diffraction and EDS. The results show that with the increase of aluminum content, the microstructure varies from FCC to FCC+BCC structure and finally to single BCC structures. And when the aluminum content is 0.5, the microstructure exhibits slender dendritic structure, then turns to mixture of eutectic structure and FCC phase at x = 0.8 and x = 1.0. when x reaches 1.5, the microstructure changes into dendritic structure. With the increase of aluminum content, the yield strength increased from 172.3 MPa to 949.6 MPa, and the hardness increased from 127 HV to 479 HV, which significantly improved the strength and hardness of the alloy.

The high-entropy alloys (HEAs)1,2) have drawn widespread attention by scientific researchers since being proposed in 2004. They are generally constituted by five principal elements, which are different from the design concept of traditional alloys based on one or two principal elements.3,4) HEAs exhibit a series of excellent properties, such as high hardness, high strength, superior thermal stability and brilliant wear resistance due to a combination of severe lattice distortion effect, sluggish diffusion effect and high entropy effect and so on.5–7) HEAs are easy to form simple solid solution structures of face-centered cube (FCC), body-centered cube (BCC) or hexagonal close-paced (HCP) attributed to their high mixing entropy. At present, most of the research work on HEAs is mainly carried out on single-phase FCC alloys. Generally speaking, the alloy containing FCC-single phase exhibits an outstanding ductility while low strength, which is exactly the opposite of BCC. Therefore, scientific researchers have designed and prepared HEAs with a dual-phase structure that has a synergistic effect of soft and hard phases according to the above mechanism. Lu et al.8,9) designed an eutectic high-entropy alloy AlCoCrFeNi2.1 integrating with an outstanding dual-phase layered FCC/B2 structure in the as-cast state and a combination of high tensile ductility and high fracture strength at room temperature by adjusting the content of nickel. Tilak Bhattacharjee10,11) and his team devised an eutectic high-entropy alloy with a strength of 1.2 GPa and a plasticity of 22.8%. Subsequently, they reported that AlCoCrFeNi2.1 has excellent tensile properties at low temperature. Li et al.12) designed the transformation-induced plasticity-assisted, FCC+HCP dual-phase Fe50Mn30Co10Cr10 high entropy alloy by controlling the ratio between the different components of the alloy to reduce the stability of solid solution and stacking fault energy, which extremely enhanced the strength and plasticity of the HEAs.
The addition of aluminum element will generate a significant impact on the lattice structure, micro-structure and mechanical properties of HEAs. Adding different content of aluminum element to AlxCoFeNi series alloys, the alloy transforms from FCC phase to BCC phase, and displays a representative dendritic micro-structure when the aluminum content increases from 0 to 1.13) Wang et al.14) prepared AlxCoCrFeNi9 (x = 0∼2) alloys and found that with the increased aluminum element, the microstructure varies from FCC to FCC+BCC structure and finally to single BCC structures and suggesting that the addition of aluminum is profitable to the formation of BCC phase in the alloy. Wang et al.15) researched the influence of the synergistic content of aluminum and copper on the microstructure and mechanical properties of Ni1.5CoFeCu1−xAlxV0.5 (x = 0.1, 0.2, 0.3) alloys. Similarly, the result of the experiment is the same as the previous study, that is to say, the increase in aluminum content promotes the growth of BCC and weakens the growth of the FCC, while the yield strength of the alloy vindicated significantly. Through the above discussion, the aluminum element will affect the structure and properties of high entropy alloy to a great extent. However, the effect of aluminum element on the phase transformation mechanism of high entropy alloy needs to be further studied. In this paper, a series of AlxCoFe1.9Ni2.1 (x = 0.5, 0.8, 1.0, 1.5) high entropy alloys were designed. And the effect of aluminum contents on microstructure and mechanical properties of the alloy was studied. From the point of view of atomic size difference (Δr) and valence electron concentration (VEC), the effect of aluminum content on the phase transition mechanism of the alloy was deeply analyzed.
The AlxCoFe1.9Ni2.1 (x = 0.5, 0.8, 1.0 and 1.5, x represents atomic proportion) HEAs with high-purity metals (≥99.95%) elements were prepared by vacuum arc melting. The alloys were directly solidified into ingots in a water-cooled Cu crucible and each ingot was re-melted 4–6 times to ensure the chemical composition homogeneity of alloys. The phase constitution of AlxCoFe1.9Ni2.1 HEAs was identified by an x-ray diffractometer (Bruker D8 Advance, Cu target) under scanning speed of 6° min−1 and scanning range of 20°∼100°, respectively. The samples were processed by mechanical polishing, and immersed in mixing acid of HNO3:HCl = 1:3 (volume ratio). The metallographic structure of the HEAs was firstly observed by optical microscope (OM). Further micro-structural characterization and component analysis were by the scanning electron microscope (SEM) equipped with back-scattered electron (BSE) and energy dispersive spectroscope (EDS). The volume fraction of different phase was investigated on SEM equipped with an electron backscatter diffraction (EBSD) detector. With the Hardness of the Vickers tester, the hardness of the alloy is measured, and each sample takes 5 points respectively, the load and duration are 1000 g and 20 s. Compression tests were conducted on a compression machine (Instron 3382) at room temperature. The size of the cylindrical samples was Φ3 × 6 mm. The strain rate of the compression tests was 5 × 10−4 s−1. To ensure the accuracy of the experiment, for each sample, at least 3 measurements were carried out.
Figure 1 shows the X-ray patterns of the as-cast AlxCoFe1.9Ni2.1 (x = 0.5, 0.8, 1.0, 1.5, hereafter referred to as Al0.5, Al0.8, Al1.0 and Al1.5, respectively) HEAs. As shown in Fig. 1, the Al0.5 alloy embodies a single FCC structure; When x attains 0.8, the BCC diffraction peak begins to appear in the diffraction spectrum, indicating that the addition of aluminum element promotes the transformation of the alloy from the single FCC phase to FCC+BCC dual phase. With the increase of aluminum content, the intensity of the diffraction peak of BCC phase increases, while the intensity of the diffraction peak of FCC phase decreases. Only BCC peak was detected when x reaches 1.5, demonstrating that the alloy phase structure has converted into a single BCC phase structure.

XRD patterns of the AlxCoFe1.9Ni2.1 alloys.
Figure 2 is a metallographic structure diagram of as-cast AlxCoFe1.9Ni2.1 alloys. It is definitely perceived that the microstructure of AlxCoFe1.9Ni2.1 alloys is formed by overlapping of dendrites. But the difference is that the lamellar second phase structures appear in the interdendrite regions when the aluminum content is 0.8 and 1.0, while the microstructure of the alloy is composed of only compact dendrites when the aluminum content is 1.5 in Fig. 2(d). Combined with Fig. 1, it can be preliminarily judged that the Al0.5 and Al1.5 high entropy alloys have a single phase structure, while the second phase forms between the dendrites in Al0.8 and Al1.0 high entropy alloys, which needs to be magnified for further observation.

Metallographical structure of as-cast AlxCoFe1.9Ni2.1 high entropy alloys. ((a) Al 0.5; (b) Al 0.8; (c) Al 1.0; (d) Al 1.5)
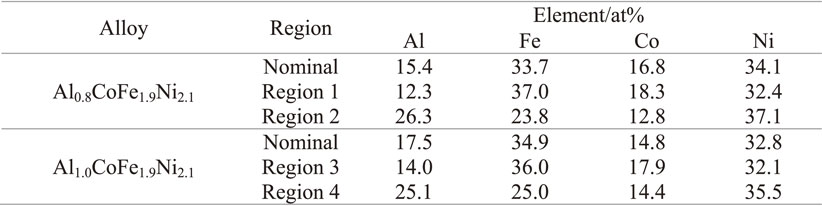
To investigate the structure of the second phase which appeared between the dendrites, the magnified SEM images of as-cast Al0.8 and Al1.0 alloys are presented in Fig. 3. The Al0.8 and Al1.0 alloys are composed of primary phase and eutectic mixture, as shown in Fig. 3. It can be obviously observed that there is a small amount of second phase between the dendrites of the Al0.8 alloy, while a large number of irregular eutectic structure appeared in the Al1.0 alloy. This show that the eutectic structure become irregular and the volume fraction of eutectic structure increases with increasing of aluminum content. In general, aluminum content of FCC phase is much less than that in BCC phase, which helps to distinguish phase types simply and effectively. The elemental compositions of primary phase and another phase in the eutectic structure of the alloy were analyzed by scanning electron microscope-energy dispersive spectroscopy (SEM-EDS). The average element content of the alloy and the element content of primary phase (1 and 3 in Fig. 3) and another phase in eutectic structure (2 and 4 in Fig. 3) is listed in Table 1. The aluminum contents in primary phases is less than 15 at%, which suggests that the primary phases are FCC phases. Simultaneously, EDS analysis results show that the primary phase is rich in iron and cobalt, while another phase in eutectic structure is rich in aluminum and nickel. This is because that aluminum-nickel has a higher negative mixing enthalpy.16) With the increase of aluminum content, it is conducive to the enrichment of aluminum and nickel atoms in interdendritic regions and the volume fraction of eutectic structure increases.

SEM images of Al0.8CoFe1.9Ni2.1 and Al1.0CoFe1.9Ni2.1 high entropy alloy. ((a) Al 0.8; (b) Al 1.0)
To investigate the variation of phase volume fraction with aluminum content, the EBSD images of as-cast AlxCoFe1.9Ni2.1 alloys are presented in Fig. 4. Among them, Fig. 4(a) shows that the Al0.5 alloy is single phase FCC structure. When x = 0.8 and 1.0, as shown in Fig. 4(b) and (c), the microstructure has changed significantly, in which the eutectic structure (FCC+BCC) appeared. From Fig. 4(b)–(d), the volume fraction of BCC phase in Al0.8, Al1.0 and Al1.5 is calculated to be 11%, 36% and 100%, respectively. It can be seen that the increasing of aluminum content promotes the form of the BCC phase. This is similar to the conclusion drawn by Zuo et al. and they thought that the structure of FeNiCoAlx gradually changes from single FCC to single BCC when X varies from 0 to 1.13) But the difference is that we found the eutectic structure composed of FCC and BCC in the alloys.

EBSD image of AlxCoFe1.9Ni2.1 high entropy alloy (Red represents FCC phase, green represents BCC phase). ((a) Al 0.5; (b) Al 0.8; (c) Al 1.0; (d) Al 1.5)
As mentioned above, the lamellar eutectic structure was discovered for the first time in FeCoNiAl high entropy alloy by adjusting alloy composition. The results show that the volume fraction of eutectic structure increases with the increase of aluminum content in a certain range. The result of EDS analysis shows that the primary phase in Al0.8 and Al1.0 alloys is FCC phase, and Al0.8 and Al1.0 alloys can be determined to be typical hypoeutectic alloys. It can be seen from Fig. 3(a) that the eutectic structure of Al0.8 alloy are less than that of Al1.0 alloy and most of the BCC phases in Al0.8 alloy exist alone in the interdendrites. This is due to the fact that the FCC phase rich in iron and cobalt crystallizes first from the melt during solidification, while aluminum and nickel are repelled into the uncrystallized melt. With the continuous formation of the primary FCC phase, the content of aluminum and nickel in the melt increases. Eutectic reaction occurs and the eutectic structure of FCC+BCC is formed when the composition of the melt reaches a certain value. In addition, due to the low content of aluminum in Al0.8, there is a large amount of primary FCC phase in the alloy. And the FCC phase in the eutectic structure tends to grow with the primary phase when the cooling rate is rapid during solidification. Finally, it leads to the combination of the primary phase and the FCC phase in the eutectic structure, and the BCC phase exists alone in the interdendrites. This phenomenon is similar as the divorced eutectic occurred in steel17) and magnesium–aluminium alloys.18)
3.2 Mechanical propertiesFigure 5 shows the effect of aluminum content on the hardness and BCC phase volume fraction of the AlxCoFe1.9Ni2.1 alloys by increasing aluminum content (x varies from 0.5 to 1.5). The hardness increases from 127 HV to 479 HV when the phase structure changes from FCC to BCC, which suggests that the hardness is closely related to the phase volume fraction of BCC structure in the alloys. Increasing the aluminum content promotes the appearance of BCC phases which is rich in aluminum and nickel elements, and significantly improves the hardness of the alloy. With the increase of the aluminum content, in addition to the sharp increase of BCC phase volume fraction, more aluminum atoms dissolve into the lattice, resulting in serious lattice distortion, which seriously hinders the movement of dislocations in the alloy and triggers the solid solution strengthening effect. Under the synergistic effect of the above two factors, the hardness of the alloy is significantly improved. The high negative mixing enthalpy of aluminum element and other elements in the alloy causes the large bonding force between atoms,19) which further hinders the movement of dislocations and improves the hardness of the alloy.

Effect of Al on hardness of AlxCoFe1.9Ni2.1 alloy and BCC phase volume change.
Compressive engineering stress-engineering strain curves of AlxCoFe1.9Ni2.1 high entropy alloys at room temperature are plotted in Fig. 6. With the increase of aluminum content (x from 0.5 to 1.5), the yield strength increases significantly from 172.3 MPa to 949.6 MPa with a reduction in the ductility. In connection with Fig. 5, the phase structure plays an important role in the compressive strength and plasticity of the material system and the performance of BCC phase in improving the compressive yield strength is better than that of FCC phase.20) It can be explained that FCC structure contains more slip systems, which improves the plastic deformation ability and reduces the strength at the same time; BCC structure shows higher strength and lower plasticity because it has less slip systems.

Compression stress-strain curve of as cast AlxCoFe1.9Ni2.1 high entropy alloys at room temperature.
The strength of Al0.8 alloy is significantly higher than that of Al0.5 alloy, which is due to the presence of a small amount of BCC phase in the alloy. With the further increase of aluminum content, it will have a serious lattice distortion effect in the alloy, which induces hindering the movement of dislocations and increases the strength of the alloy. At the same time, aluminum and nickel elements is enriched in the inter-dendrite regions to form eutectic structure, distributed in layers between the dendrites, which greatly expands the area of the phase boundary.21) The appearance of eutectic structure results in the increase of phase boundary area, which seriously hinders the movement of dislocations, slows down the slip of dislocations and improves the mechanical properties of the alloy.22) As can be seen from Fig. 6, when x reaches 1.5, the solid solution strengthening effect of the alloy is very significant. The negative mixing enthalpy of aluminum and other elements promotes a large bonding force between the atoms, and the alloy is all composed of hard BCC phase, which is the reason for the significant improvement of the strength of Al1.5CoFe1.9Ni2.1 alloy.
The above experimental results indicate that the aluminum element addition significantly affects the microstructure of the AlxCoFe1.9Ni2.1 HEAs. With the aluminum element addition, the volume fraction of FCC phase decreased while the volume fraction of BCC phases increased, as shown in Fig. 5. This result might be explained by that as a foreign element, the large atomic size difference (Δr) between aluminum and other elements could induce serious lattice distortion, which promote the disintegration of close-packed FCC structure and the formation of a loose-packed structure.23) The atomic size difference in an alloy is quantified by δ:24)
| \begin{equation} \delta = \sqrt{\sum\nolimits_{i=1}^{n}c_{i}\left(1 - \frac{r_{i}}{{\bar{r}}}\right)^{2}},\ \bar{r} = \sum\nolimits_{i=1}^{n}c_{i}r_{i} \end{equation} | (1) |

Variation of BCC phase volume and atomic size difference as a function of Al content in AlxCoFe1.9Ni2.1 high entropy alloys.
In addition to the atomic size difference, the valence electron concentration (VEC) as another empirical parameter will affect the phase stability of HEAs26) which can be expressed by following equation (2):
| \begin{equation} \mathit{VEC} = \sum_{i=1}^{n}c_{i}(\mathit{VEC})_{i} \end{equation} | (2) |
To correlate the mechanical properties with the crystal structure, variation of compressive yield strength (σy) and micro-hardness (HV) as a function of VEC for the AlxCoFe1.9Ni2.1 HEAs are demonstrated in Fig. 8. Both yield strength (σy) and micro-hardness (HV) increased with the VEC value decreased. In the AlxCoFe1.9Ni2.1 HEA system, the Al1.5 alloy with a lowest VEC value possessed highest strength and hardness. The Al0.5 alloy has a single-phase FCC structure, possessing a higher VEC value, displayed lower yield strength, but better ductility then other three alloys, as shown in Fig. 8. This is mainly due to the decrease of VEC value, which leads to the unstable disintegration of FCC phase and promotes the formation of hard BCC phase, thus significantly improving the yield strength and hardness of AlxCoFe1.9Ni2.1 high entropy alloy.

Variation of compressive yield strength and micro-hardness as a function of VEC of the AlxCoFe1.9Ni2.1 high entropy alloys.
In this work, the effect of aluminum content on microstructural evolution and mechanical behavior of the as-cast AlxCoFe1.9Ni2.1 (x = 0.5, 0.8, 1.0, 1.5) high entropy alloys were studied systematically. The main conclusions are drawn as follows:
This work was financially supported by the General Program of National Nature Science Foundation of China (No. 51974134); Major Science and Technology Special Project of Hebei Province (21281008Z); Key Program of Science and Technology of Universities in Hebei Province (ZD2016019).